Practice Essentials
A toxic nodular goiter (TNG) is a thyroid gland that contains autonomously functioning thyroid nodules, with resulting hyperthyroidism. There are distinct considerations if the patient has a single solitary toxic nodule (see Solitary Thyroid Nodule). TNG, or Plummer disease, was first described by Henry Plummer in 1913. TNG is the second most common cause of hyperthyroidism in the Western world, after Graves disease. In elderly individuals and in areas of endemic iodine deficiency, TNG is the most common cause of hyperthyroidism.
Signs and symptoms
Most patients with TNG present with symptoms typical of hyperthyroidism, including the following:
-
Heat intolerance
-
Palpitations
-
Tremor
-
Weight loss
-
Hunger
-
Frequent bowel movements
Physical examination findings of hyperthyroidism may be more subtle than those of Graves disease. Features may include the following:
-
Widened palpebral fissures
-
Tachycardia
-
Hyperkinesis
-
Moist, smooth skin
-
Tremor
-
Proximal muscle weakness
-
Brisk deep tendon reflexes
See Presentation for more detail.
Diagnosis
Laboratory studies
Third-generation thyroid-stimulating hormone (TSH) assays are generally the best initial screening tool for hyperthyroidism. Patients with TNG will have suppressed TSH levels.
Imaging studies
Patients with biochemical hyperthyroidism should undergo nuclear scanning. In patients with TNG, the scan results usually reveal patchy uptake, with areas of increased and decreased uptake. (See the image below.)
Ultrasonography is a highly sensitive procedure for delineating discrete nodules that are not palpable during thyroid examination.
See Workup for more detail.
Management
The optimal therapy for TNG remains controversial and differs slightly between a single dominant nodule and multiple toxic nodules. Patients who have autonomously functioning nodules should be treated definitively with radioactive iodine or surgery. Those with subclinical hyperthyroidism should be monitored closely for overt disease.
Antithyroid drugs and beta blockers are used for short courses in the treatment of TNG; they are important in rendering patients euthyroid in preparation for radioiodine or surgery and in treating hyperthyroidism while awaiting a full clinical response to radioiodine.
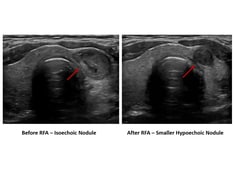
When radioactive iodine, surgery, or long-term antithyroidal drugs are inappropriate or contraindicated, radiofrequency ablation can be considered in select patients.
See Treatment and Medication for more detail.
Pathophysiology
Toxic nodular goiter (TNG) represents a spectrum of disease ranging from a single hyperfunctioning nodule (toxic adenoma) within a multinodular thyroid to a gland with multiple areas of hyperfunction. The natural history of a multinodular goiter involves variable growth of individual nodules; this may progress to hemorrhage and degeneration, followed by healing and fibrosis. Calcification may be found in areas of previous hemorrhage. Some nodules may develop autonomous function. Autonomous hyperactivity is conferred by somatic mutations of the thyrotropin, or thyroid-stimulating hormone (TSH), receptor in 20-80% of toxic adenomas and some nodules of multinodular goiters. [1] Autonomously functioning nodules may become toxic in 10% of patients. Hyperthyroidism predominantly occurs when single nodules are larger than 2.5 cm in diameter. Signs and symptoms of TNG are similar to those of other types of hyperthyroidism.
Etiology
Functional autonomy of the thyroid gland appears to be related to iodine deficiency. Various mechanisms have been implicated, but the molecular pathogenesis is poorly understood.
The sequence of events leading to toxic multinodular goiter is as follows:
-
Iodine deficiency leads to low levels of T4; this induces thyroid cell hyperplasia to compensate for the low levels of T4.
-
Increased thyroid cell replication predisposes single cells to somatic mutations of the TSH receptor. Constitutive activation of the TSH receptor may generate autocrine factors that promote further growth, resulting in clonal proliferation. Cell clones then produce multiple nodules.
Somatic mutations of the TSH receptors and G α protein confer constitutive activation to the cyclic adenosine monophosphate (cAMP) cascade of the inositol phosphate pathways. These mutations may be responsible for functional autonomy of the thyroid in 20-80% of cases. [1]
These mutations are found in autonomously functioning thyroid nodules, solitary and within a multinodular gland. Nonfunctioning thyroid nodules within the same gland lack these mutations.
The reported frequency of these mutations varies widely, ranging from 10-80%. Higher incidence is reported in patients with iodine deficiency.
In addition to somatic mutations, polymorphisms of the TSH receptor have been studied in patients with toxic nodular goiter (TNG); notably, polymorphisms involving the carboxyl-terminal tail of the human TSH receptor have been found in nodular and genomic deoxyribonucleic acid (DNA).
Unlike the somatic mutations found in autonomously functioning nodules, these mutations have also been found in other cell lines, indicating a germline mutation. One of these, D727E, was present with greater frequency in patients with TNG than in healthy individuals; this suggests that this polymorphism may be associated with the disease. [2, 3]
The presence of the heterozygous state for the D727E variant of the human TSH receptor alone is not sufficient for the development of the TNG. Approximately 10% of healthy individuals have this polymorphism.
Possible mediators in growth include the following:
-
Endothelin-1 (ET-1) production is increased in rat thyroid glands that have undergone hyperplasia; this suggests that ET-1 production may be involved in thyroid gland growth and vascularity. In contrast to normal thyroid tissue and papillary thyroid cancer, thyroid tissue in patients with TNG shows markedly positive staining of the stroma but absent staining of the follicular cells. The significance of this finding is unclear, but ET-1 is, in addition to being a vasoconstrictor, a mitogen for vascular endothelium, smooth muscle cells, and thyroid follicular cells.
-
In vitro systems have shown stimulation of thyroid follicular cell proliferation with insulinlike growth factor-1, epidermal growth factor, and fibroblast growth factor. Reduced concentrations of transforming growth factor-β 1 or resistance to transforming growth factor-β have also been associated with follicular cell growth. The role of these multiple factors in the growth and secretory function of TNG needs further investigation.
Epidemiology
United States statistics
Toxic nodular goiter accounts for approximately 15-30% of cases of hyperthyroidism in the United States, second only to Graves disease.
International statistics
In areas of endemic iodine deficiency, toxic nodular goiter (TNG) accounts for approximately 58% of cases of hyperthyroidism, 10% of which are from solitary toxic nodules. Graves disease accounts for 40% of cases of hyperthyroidism. In patients with underlying nontoxic multinodular goiter, initial iodine supplementation (or iodinated contrast agents) can lead to hyperthyroidism (Jod-Basedow effect). Iodinated drugs, such as amiodarone, may also induce hyperthyroidism in patients with underlying nontoxic multinodular goiter. Roughly 3% of patients treated with amiodarone in the United States (more in areas of iodine deficiency) develop amiodarone-induced hyperthyroidism. [4]
Sex- and age-related demographics
Toxic nodular goiter occurs more commonly in women than in men. In women and men older than 40 years, the prevalence rate of palpable nodules is 5-7% and 1-2%, respectively.
Most patients with toxic nodular goiter (TNG) are older than 50 years.
Thyrotoxicosis often occurs in patients with a history of longstanding goiter. Toxicity occurs in a subset of patients who develop autonomous function. This toxicity usually peaks in the sixth and seventh decades of life, especially in persons with a family history of multinodular goiter or TNG, suggesting a genetic component.
Prognosis
Most treated patients have a good prognosis. A worse prognosis is related to untreated hyperthyroidism. Patients should understand the gravity of hyperthyroidism. If left untreated, hyperthyroidism may lead to osteoporosis, arrhythmia, heart failure, coma, and death. Regular assessment of thyroid function is important in monitoring disease.
Na131 I ablation may result in continued hyperthyroidism, with some patients (up to 73% in some studies, depending on the size of the goiter and the dosing of radioiodine) requiring repeated treatment or surgical removal of the gland. Hypothyroidism after radioiodine ablation has been reported in 0-35% of individuals.
Iodine-131 ablation may result in continued hyperthyroidism, with some patients (up to 73% in some studies depending on size of goiter and dosing of radioiodine) requiring repeated treatment or surgical removal of the gland. Hypothyroidism after radioiodine ablation has been reported in 0-35% of individuals.
Surgical treatment usually consists of a lobectomy of the hyperfunctioning nodule. The rate of hypothyroidism associated with this procedure is very low. Rates of hyperthyroidism recurrence with surgery have been reported to be as low as 0-9%. Larger, multinodular goiters may require total thyroidectomy.
Morbidity/mortality
Morbidity and mortality from toxic nodular goiter (TNG) may be divided into problems related to hyperthyroidism and problems related to growth of the nodules and gland. Local compression problems due to nodule growth, although unusual, include dyspnea, hoarseness, and dysphagia. Both TNG and Graves disease have increased mortality but for different reasons. [5]
TNG is more common in elderly adults; therefore, complications due to comorbidities, such as coronary artery disease, are significant in the management of hyperthyroidism.
Hyperthyroid complications
The most important complications are related to the heart.
Cardiomyopathy resulting in severely depressed function may be observed with hyperthyroidism, possibly in relation to persistent tachycardia. Fortunately, cardiomyopathy resolves remarkably with resolution of the hyperthyroid state.
Using anticoagulants to treat patients exhibiting atrial fibrillation remains controversial, although it is recommended by many authorities. Atrial fibrillation of long duration that is associated with other anatomical defects of the heart should be treated with warfarin or another suitable anticoagulant.
Patient Education
Many patients fear abnormal weight gain with the attainment of the euthyroid state. Provide patients with education regarding the role of thyroid hormone in metabolism, as well as the cardiovascular and thromboembolic risks of hyperthyroidism. Also provide guidelines for lifestyle modification that will allow the patient to avoid weight gain.
Apprise patients treated with PTU or methimazole of the risk of agranulocytosis and instruct them to contact a physician if they develop a fever, rash, or sore throat, so that a complete blood cell (CBC) count with differential can be urgently performed.
-
Patchy uptake of iodine (123I) in a toxic multinodular goiter.