Practice Essentials
Crohn disease is an idiopathic, chronic inflammatory process that can affect any part of the gastrointestinal tract from the mouth to the anus (see the image below). Individuals with this condition often experience periods of symptomatic relapse and remission.
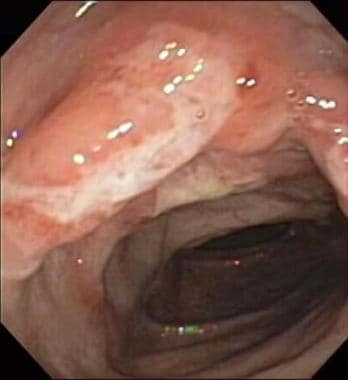 Crohn disease. The colonoscopy image reveals a large ulcer and inflammation of the descending colon in a 12-year-old boy with Crohn disease.
Crohn disease. The colonoscopy image reveals a large ulcer and inflammation of the descending colon in a 12-year-old boy with Crohn disease.
See Autoimmune Disorders: Making Sense of Nonspecific Symptoms, a Critical Images slideshow, to help identify several diseases that can cause a variety of nonspecific symptoms.
Signs and symptoms
The characteristic presentation in Crohn disease is abdominal pain and diarrhea, which may be complicated by intestinal fistulization or obstruction. Unpredictable flares and remissions characterize the long-term course. [1, 2, 3]
Other signs and symptoms of Crohn disease may include the following:
-
Rectal bleeding
-
Fever
-
Weight loss, anorexia
-
Nausea, vomiting
-
Malnutrition, vitamin deficiencies
-
Generalized fatigability
-
Bone loss
-
Growth failure in pediatric patients: May precede gastrointestinal symptoms by years
See Clinical Presentation for more detail.
Diagnosis
Examination for Crohn disease includes the following:
-
Vital signs: Normal, but possible presence of tachycardia in anemic or dehydrated patients; possible chronic intermittent fever
-
Gastrointestinal: May vary from normal to those of an acute abdomen; assess for rectal sphincter tone, gross rectal mucosal abnormalities, presence of hematochezia
-
Genitourinary: May include presence of skin tags, fistulae, ulcers, abscesses, and scarring in the perianal region; nephrolithiasis, hydronephrosis, and enterovesical fistulae
-
Musculoskeletal: Possible arthritis and arthralgia, particularly of the large joints [6]
-
Dermatologic: May show pallor or jaundice, mucocutaneous or aphthous ulcers, erythema nodosum, and pyoderma gangrenosum
-
Ophthalmologic: May reveal episcleritis; possible uveitis
-
Growth delay: Decreased growth velocity (eg, height), pubertal delay
-
Hematologic: Hypercoagulable state
Laboratory Tests
Although laboratory results for Crohn disease are nonspecific and are of value principally for facilitating disease management, they may also be used as surrogate markers for inflammation and nutritional status and to screen for deficiencies of vitamins and minerals.
Routine laboratory studies include the following:
-
CBC count
-
Chemistry panel
-
Liver function tests
-
Inflammatory markers
-
Stool studies
-
Serologic tests
Imaging studies
Imaging modalities used for Crohn disease include the following:
-
Plain abdominal radiography
-
Barium contrast studies (eg, small bowel follow-through, barium enema, enteroclysis)
-
CT scanning of the abdomen
-
CT enterography or magnetic resonance enterography: Replacing small bowel follow-through studies
-
MRI of the pelvis
-
Abdominal and/or endoscopic ultrasonography
-
Nuclear imaging (eg, technetium-99m hexamethyl propylene amine oxime, indium-111)
-
Fluorine-18-2-fluoro-2-deoxy-D-glucose scanning combined with positron emission tomography or CT scanning
Procedures
The following procedures may help in the evaluation of Crohn disease:
-
Endoscopic visualization and biopsy (eg, upper gastrointestinal endoscopy, esophagogastroduodenoscopy, endoscopic retrograde cholangiopancreatography)
-
Colonoscopy, ileocolonoscopy
-
Small bowel enteroscopy
-
Interventional radiology: For percutaneous drainages of abscesses
See Workup for more detail.
Management
Pharmacotherapy
Medications used in the treatment of Crohn disease include the following:
-
5-Aminosalicylic acid derivative agents (eg, mesalamine rectal, mesalamine, sulfasalazine, balsalazide)
-
Corticosteroids (eg, prednisone, methylprednisolone, budesonide, hydrocortisone, prednisolone)
-
Immunosuppressive agents (eg, mercaptopurine, methotrexate, tacrolimus)
-
Monoclonal antibodies (eg, infliximab, adalimumab, certolizumab pegol, natalizumab, ustekinumab, vedolizumab)
-
Antibiotics (eg, metronidazole, ciprofloxacin)
-
Antidiarrheal agents (eg, loperamide, diphenoxylate-atropine)
-
Bile acid sequestrants (eg, cholestyramine, colestipol)
-
Anticholinergic agents (eg, dicyclomine, hyoscyamine, propantheline)
Surgery
Unlike ulcerative colitis, Crohn disease has no surgical cure. Most patients with Crohn disease require surgical intervention during their lifetime.
Surgical management of the terminal ileum, ileocolon, and/or upper gastrointestinal tract may include the following [7] :
-
Resection of the affected bowel
-
Ileocolostomy or proximal loop ileostomy
-
Drainage of any septic foci with later definitive resection
-
Strictureplasty
-
Bypass
-
Endoscopic dilatation of symptomatic, accessible strictures
Surgical management of the colon may include the following [7] :
-
Subtotal or total colectomy with end ileostomy (laparoscopic or open approach)
-
Segmental or total colectomy with or without primary anastomosis
-
Total proctocolectomy or proctectomy with stoma creation
See Treatment and Medication for more detail.
Background
Crohn disease is an idiopathic, chronic inflammatory process of the gastrointestinal (GI) tract that can affect any part of the tract from the mouth to the anus. Individuals with this condition often experience periods of symptomatic relapse and remission.
Crohn disease is believed to be the result of an imbalance between proinflammatory and anti-inflammatory mediators. Although genetic susceptibility, luminal antigenic drive, and environmental triggers are also important factors, animal models demonstrate that no single factor is sufficient to induce intestinal inflammation. (See Pathophysiology.)
Approximately 30% of Crohn disease cases involve the small bowel, particularly the terminal ileum, another 20% involve only the colon, and 45% involve both the small bowel and colon. [8] Once considered rare in the pediatric and black populations, Crohn disease is recognized with increasing frequency in children of all ages and in individuals of varying ethnicities. (See Epidemiology.)
The characteristic presentation is abdominal pain and diarrhea, which may be complicated by intestinal fistulization or obstruction. Unpredictable flares and remissions characterize the long-term course. [1, 2, 3] In addition, individuals can experience rectal bleeding, fever, weight loss, malnutrition, bone loss, and vitamin deficiencies. Psychosocial issues (eg, depression, anxiety, and coping difficulty) are common. Pediatric patients may also experience psychological issues regarding quality of life and body image. [4, 5] (See Presentation.)
Laboratory data for Crohn disease are nonspecific and are of value principally in assisting with management. However, various imaging modalities can aid in diagnosis and management; the choice among them depends upon the clinical question being asked. (See Workup.)
Plain radiography or computed tomography (CT) of the abdomen and pelvis can assess for bowel obstruction or pelvic intra-abdominal abscesses. Small bowel follow-through (SBFT) studies are being supplanted by CT enterography or magnetic resonance (MR) enterography, which is better able to distinguish inflammation from fibrosis. Magnetic resonance imaging (MRI) of the pelvis or endoscopic (transrectal) ultrasonography can identify perianal fistula anatomy and activity and determine the presence or absence of pelvic and perianal abscesses.
Endoscopic visualization and biopsy are essential in the diagnosis of Crohn disease. Colonoscopy is done to assess for colonic or terminal ileal disease. Upper GI endoscopy may be used to diagnose esophageal or gastroduodenal disease and is recommended for all children, regardless of the presence or absence of upper GI symptoms. (See Workup.)
The general goals of treatment are as follows:
-
To achieve the best possible clinical, laboratory, and histologic control of the inflammatory disease with the least adverse effects from medications
-
To permit the patient to function as normally as possible
-
In children, to promote growth with adequate nutrition; the unique problems encountered in the pediatric population necessitate a medical approach that promotes clinical improvement and reverses growth failure with minimal toxicity
Therapy is typically administered in a “step-up” approach, in which patients with mild disease are treated with 5-aminosalicylic acid (5-ASA), antibiotics, and nutritional therapy. If the patient does not respond to this approach or if the disease is more severe than was initially thought, corticosteroid and immunomodulatory therapy with 6-mercaptopurine (6-MP) or methotrexate is attempted. Finally, biologic and surgical therapies, at the tip of the treatment pyramid, are used. [9] (See Treatment.)
A subpopulation of patients with risk factors for complicated disease and rapid progression may benefit from a “top-down” approach. This approach involves early and aggressive use of tumor necrosis factor (TNF) antagonists, which may alter the natural history of the disease, improve treatment response, and decrease the need for steroid therapy. (See Treatment.)
Surgery plays an integral role in controlling medically refractory disease and treating complications of Crohn disease. Because of the high rate of disease recurrence after segmental bowel resection, the guiding principle of surgery is preservation of intestinal length and function. [1] (See Treatment.)
Pathophysiology
Chronic inflammation from T-cell activation leading to tissue injury is implicated in the pathogenesis of Crohn disease.
After activation by antigen presentation, unrestrained responses of type 1 T helper (Th1) cells predominate in Crohn disease as a consequence of defective regulation. Th1 cytokines such as interleukin (IL)-12 and TNF-α stimulate the inflammatory response. Inflammatory cells recruited by these cytokines release nonspecific inflammatory substances, including arachidonic acid metabolites, proteases, platelet activating factor, and free radicals, which result in direct injury to the intestine.
In a study from 2012, investigators suggested that genetic predispositions for inflammatory bowel disease (IBD) lead to abnormal epithelial barrier integrity and homeostasis, deficits in autophagy, deficiencies in innate pattern recognition receptors, and problems with lymphocyte differentiation, especially in Crohn disease. [10]
Microscopically, the initial lesion starts as a focal inflammatory infiltrate around the crypts, followed by ulceration of superficial mucosa. Later, inflammatory cells invade the deep mucosal layers and, in that process, begin to organize into noncaseating granulomas (see the image below). The granulomas extend through all layers of the intestinal wall and into the mesentery and the regional lymph nodes.
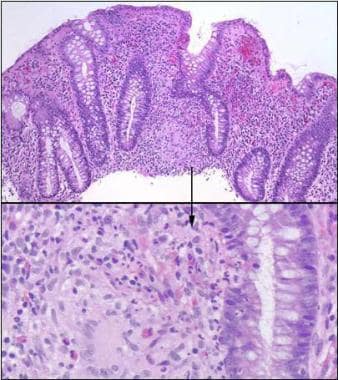 Crohn disease. Colonic granuloma in a patient with Crohn disease is shown (hematoxylin-eosin staining). Image courtesy of Dr E. Ruchelli.
Crohn disease. Colonic granuloma in a patient with Crohn disease is shown (hematoxylin-eosin staining). Image courtesy of Dr E. Ruchelli.
Neutrophil infiltration into the crypts forms crypt abscesses, leading to destruction of the crypt and atrophy of the colon. Chronic damage may be seen in the form of villous blunting in the small intestine as well. Ulcerations are common and are often seen on a background of normal mucosa.
Although granuloma formation is pathognomonic of Crohn disease, its absence does not exclude the diagnosis. [11]
Macroscopically, the initial abnormality consists of hyperemia and edema of the involved mucosa. Later, discrete superficial ulcers form over lymphoid aggregates and are seen as red spots or mucosal depressions (see the image below). These can become deep, serpiginous ulcers located transversely and longitudinally over an inflamed mucosa, giving the mucosa a cobblestone appearance. The lesions are often segmental, being separated by healthy areas, and are referred to as skip lesions. [11]
 Crohn disease. The colonoscopy image reveals a large ulcer and inflammation of the descending colon in a 12-year-old boy with Crohn disease.
Crohn disease. The colonoscopy image reveals a large ulcer and inflammation of the descending colon in a 12-year-old boy with Crohn disease.
Transmural inflammation results in thickening of the bowel wall and narrowing of the lumen. As Crohn disease progresses, it is complicated by obstruction or deep ulceration leading to fistulization by way of the sinus tracts penetrating the serosa, microperforation, abscess formation, adhesions, and malabsorption. [1]
Bowel obstruction is caused initially by significant edema of the mucosa and associated spasm of the bowel. Obstruction is intermittent and can often be reversed by means of conservative measures and anti-inflammatory agents. With further disease progression, the obstruction becomes chronic because of fibrotic scarring, luminal narrowing, and stricture formation. [1]
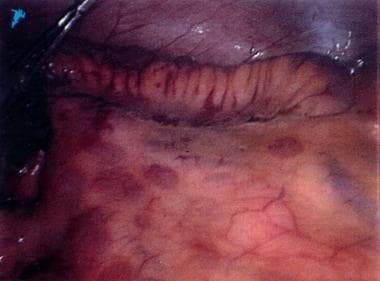
Fistulae may be enteroenteral, enterovesical, enterovaginal, or enterocutaneous. The inflammation extending through the bowel wall may also involve the mesentery and surrounding lymph nodes. Creeping fat may be seen when the mesentery wraps around the bowel surface (see the following image). [1] Serosal inflammation causes adhesions; thus, free perforations are less common in Crohn disease than in other inflammatory bowel conditions. [1]
Etiology
The exact cause of Crohn disease remains unknown. Genetic, microbial, immunologic, environmental, dietary, vascular, and psychosocial factors have been implicated, as have smoking and the use of oral contraceptives and nonsteroidal anti-inflammatory agents (NSAIDs). [12] Patients may inherit susceptibility for an aberrant immunologic response to 1 or more of these provoking factors. [11] Interaction between the predisposing genetic factors, environmental factors, host factors, and triggering event is likely necessary for the disease to develop.
Studies have found compelling evidence for an inheritable risk for the development of Crohn disease. However, classic mendelian inheritance is not seen. Most of the genes thought to be involved in the development of the disease play a role in mucosal immunity, and their products are found on the mucosal barrier epithelium. [11]
When the genetics of Crohn disease were first investigated, a strong association was found with chromosome 16 (IBD1 gene), which led to the identification of 3 single nucleotide polymorphisms (SNPs), 2 missense and 1 frameshift, in the NOD2 gene (now called CARD15), the first gene clearly identified as a susceptibility gene for Crohn disease.
NOD2/CARD15 is a polymorphic gene involved in the innate immune system. Of its more than 60 variations, 3 play a role in 27% of patients with Crohn disease, primarily in those with ileal disease. Subsequent studies suggest that CARD15 genotype is associated not only with the onset of disease but also with its natural history. A study in a German and Norwegian cohort showed that patients with 1 of the 3 identified risk alleles for CARD15 were more likely to have either ileal or right-colon disease. [13, 14]
Another early genome-wide association study (GWAS) looked at Jewish and non-Jewish case-control cohorts and identified 2 SNPs in the IL23R gene, which encodes 1 subunit of the IL-23 receptor protein. [15] Interestingly, this study also described the promising nature of certain therapies that block the function of IL-23. Further research suggested that one particular polymorphism in the IL23R gene showed the strongest association in a German population. [16]
However, another study found that the Arg381Gln substitution is associated with childhood onset of IBD in Scotland. [17] Numerous other loci have been identified as conferring susceptibility to Crohn disease. Several large studies found multiple susceptibility loci and confirmed earlier findings.
In a meta-analysis of 3 GWASs, 526 SNPs from 74 distinct genomic loci were found. [18] In addition to loci that have been previously discussed, 21 new loci were found that were associated with an increased risk of developing Crohn disease. Among the new loci were some very interesting implications, including the genes CCR6, IL12B, STAT3, JAK2, LRRK2, CDKAL1, and PTPN22. [18] Most of these genes are involved in signal transduction in certain immune function, as well as genes involved more directly with immune function.
The interlectin gene (ITLN1) is expressed in the small bowel and colon and is involved in recognition of certain microorganisms in the intestine. Other GWASs found associations between susceptibility to Crohn disease and polymorphisms in genes associated with the intestinal milieu. One study, involving nearly 20,000 SNPs in 735 individuals with Crohn disease, found an association in the ATG16L1 gene, which encodes the autophagy-related 16-like protein involved in the autophagosome pathway that processes intracellular bacteria. [19, 20]
SNPs in other autophagy genes have also been associated with susceptibility to Crohn disease, as in one study examining at 2 polymorphisms that flanked the IRGM gene and that may be in the regulatory material for the gene. [21] Subsequently, various other loci have been implicated in the autophagy pathway as being associated with Crohn disease, with mounting evidence that the autophagosome pathway is very important in the pathogenesis of the disease.
Studies have also provided strong support for IBD susceptibility genes on chromosome 5p13.1, which is a gene desert but does modulate expression of the PTGER4 gene. A murine PTGER4 knockout model has been studied and found to exhibit significant susceptibility to severe colitis. [22]
A large genomic study of multiple diseases confirmed many of the findings found in earlier studies and identified several additional loci of interest for Crohn disease. [23, 24] A locus at 3p21 is located within the BSN gene, which encodes a brain-specific scaffold protein involved in neurotransmitter release. However, the MST1 gene is located nearby and encodes a macrophage stimulation gene, and the authors felt that this represented a more plausible explanation for the association. [24]
A locus at 10q24.2 is located near the NKX2-3 gene, which is a homeodomain-containing transcription factor.
Disruption of the homologous gene in a murine model resulted in defective development of the intestine. [25] The investigators hypothesized that changes to expression of this gene could alter the migration of lymphocytes in the intestine and change its inflammatory response. The last locus discussed in this model is immediately upstream of the PTPN2 on chromosome 18p11 and encodes a T cell protein tyrosine phosphatase, which is a negative regulator of inflammation. [25]
Infectious agents such as Mycobacterium paratuberculosis, Pseudomonas species, and Listeria species have all been implicated in the pathogenesis of Crohn disease, suggesting that the inflammation seen with the disease is the result of a dysfunctional, but appropriate, response to an infectious source. [11]
Interleukins and TNF-α have also been implicated in the disease process. Crohn disease is characterized by a Th1 cellular immune response pattern that leads to production of IL-12, TNF-α, and interferon gamma. TNF-α has been shown to play a critical role in the inflammation in this disease. Increased production of TNF-α by macrophages in patients with Crohn disease results in increased concentrations of TNF-α in the stool, blood, and mucosa. [26]
Environmental influences such as tobacco use seem to have an effect on Crohn disease. Smoking has been shown to double the risk of Crohn disease, whereas the risk of developing ulcerative colitis is lower in people who smoke than in those who have never smoked or in those who stopped smoking before their diagnosis. [11, 27]
It has been suggested that a diet high in fatty foods may increase the risk of Crohn disease. [28] Concerns about the measles vaccine and the development of the disease have proved to be unfounded. [29] Although appendectomy has been suggested to be protective in ulcerative colitis, it is not a protective factor in Crohn disease. [30]
Epidemiology
United States statistics
In 1998, the prevalence of Crohn disease in the United States was estimated on the basis of data from Olmstead County, Minnesota, and was approximated at 8 cases per 100,000 population. [31] A subsequent analysis of a geographically diverse health insurance claims database estimated the prevalence of Crohn disease among US children and adults in 2003-2004 to be closer to 201 cases per 100,000 persons among adults and 43 per 100,000 among children. [32]
Urban areas may have a higher prevalence of IBD than rural areas do. [1, 2] Upper socioeconomic classes are thought to have a higher prevalence than lower socioeconomic classes, a difference that is likely influenced by increased access to health care, though genetic and environmental factors may also play a role. [1, 6]
International statistics
Within Europe and North America, a north-to-south gradient in the frequency of IBD in populations is present. This difference in incidence correlates with the highest frequency of IBD in temperate climates and more industrialized parts of the world, such as Western Europe and North America. [33] As new regions assume Western cultural practices, an increased prevalence of ulcerative colitis is usually found approximately 1 decade before the observed increase in Crohn disease.
The overall incidence of Crohn disease in Europe is about 5.6 per 100,000 inhabitants (7.0 per 100,000 person-years in northern centers vs 3.9 in southern centers). [34] In most Western European countries, the incidence has stabilized or slightly increased. Increases are reported from some high-incidence areas (eg, Denmark and Sweden). Earlier studies from the 1980s reported an incidence of 4.1 per 100,000 person-years, whereas data for 2003-2005 indicate an incidence of 8.6 per 100,000 person-years. [35]
Incidence figures in Asia range from 0.5 to 4.2 cases per 100,000 persons. [36] In Japan, there are about 40,000 patients with Crohn disease (approximately 27 per 100,000 persons). [12] The lowest recorded rates of new cases appear to be in South Africa (0.3-2.6 cases per 100,000 persons) and Latin America (0-0.03 cases per 100,000 persons). [1, 2]
A systematic review revealed that the highest prevalence for Crohn disease in North America was 319 per 100,000 persons, compared with 322 per 100,000 persons in Europe. [37] The highest annual incidence figures were 20.2 per 100,000 person-years in North America, 12.7 per 100,000 person-years in Europe, and 5.0 per 100,000 person-years in Asia and the Middle East. In time-trend analyses, 75% of the epidemiologic studies showed statistically significant increases in the incidence of Crohn disease over time. [37]
Age-, sex-, and race-related demographics
The age of onset of Crohn disease has a bimodal distribution. The first peak occurs between the ages of 15 and 30 years (late adolescence and early adulthood), and the second occurs mainly in women between the ages of 60 and 70 years. However, most cases begin before age 30 years, and approximately 20-30% of all patients with Crohn disease are diagnosed before age 20 years. A greater proportion of colonic and distal Crohn disease has been diagnosed in older patients, whereas younger patients have predominantly ileal disease. [1]
In general, the frequency of IBD is similar in males and females, with some studies showing a very slight female predominance. The rate of Crohn disease is 1.1-1.8 times higher in women than in men. [38] This pattern is reversed with pediatric IBD, which has a higher incidence in boys than in girls (pediatric male-to-female ratio, ~1.6:1). In Japan, a male predominance exists. [12]
Crohn disease is reported to be more common in white patients than in black patients and rare in Asian and Hispanic children. Approximately 20% of all IBD patients are of black descent. Rates are higher in people of Jewish descent, particularly in Ashkenazi Jews and Jews of middle European origin as compared with Sephardic or eastern European Jews. [39]
Prognosis
Crohn disease is a chronic inflammatory condition with an indolent course. Appropriate medical and surgical therapy helps patients to have a reasonable quality of life, with an overall good prognosis and an extremely low risk of a fatal outcome. [1]
Several earlier studies estimated a slight decrease in life expectancy associated with certain prognostic indicators, such as female sex, long disease duration, and disease location. The increased mortality was related to pulmonary malignancies, genitourinary tract diseases, and GI, liver, and biliary diseases.
In contrast, other studies have reported normal survival in patients with Crohn disease. With the advent of new medical therapies, population-based studies have shown that overall survival for North American patients with IBD is similar to that expected in the US white population. [40] Individuals with Crohn disease were at increased risk of death from complications of GI disease, GI malignancy, and chronic obstructive pulmonary disease (COPD). [40]
In a Danish study that evaluated trends in mortality from 1982 to 2010, investigators observed a 50% higher mortality in patients with Crohn disease relative to the general population; this percentage did not change over time. [41]
Crohn disease is typically characterized by periods of remission and relapse. In the first year after diagnosis, the relapse rate approaches 50%, with 10% of patients having a chronic relapsing course. [1] Most patients develop complications that require surgery, and postoperative clinical relapse occurs in a significant proportion. [1] The risk of surgery at 5-year intervals after diagnosis is as follows [42] :
-
5 years after diagnosis – The cumulative probability of having only 1 surgical procedure is 37%; 2 or more surgical procedures, 12%; and no surgical procedures, 51%
-
10 years after diagnosis – The cumulative probability of having only 1 surgical procedure is 39%; 2 or more surgical procedures, 23%; and no surgical procedures, 39%
-
15 years after diagnosis – The cumulative probability of having only 1 surgical procedure is 34%; 2 or more surgical procedures, 36%; and no surgical procedures, 30%
Patients with proximal small bowel disease have a higher risk of mortality than those who have ileal or ileocecal disease. The excess mortality may be ascribed to complications of Crohn disease. [1]
Acute Crohn disease of the terminal ileum is often discovered during laparotomy for suspected appendicitis and has an excellent prognosis. The acute episode is usually treated conservatively, and as many as two thirds of patients may show no subsequent evidence of regional enteritis. [1]
Discussion of the diagnosis, management, and surveillance of colorectal cancer in patients with IBD is beyond the scope of this article. Current data suggest that with the advent of improved therapies for patients with IBD, there is a trend toward decreasing risk of colorectal cancer. For more information, see the following 2 guidelines:
-
AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. 2010. Available at: http://www.gastrojournal.org/article/S0016-5085(09)02202-1/fulltext. Accessed September 11, 2012
-
Colonoscopic surveillance for prevention of colorectal cancer in people with ulcerative colitis, Crohn disease or adenomas. London, UK: National Institute for Health and Clinical Excellence (NICE); 2011. Available at: http://guideline.gov/content.aspx?id=34830. Accessed September 11, 2012
Genetic studies are yielding evidence associating particular variants of the CARD15 gene with the prognosis of Crohn disease. [10] Specific CARD15 mutations have been linked with the intestinal site of the disease (eg, ileal site), and certain variants have been found to be associated with the propensity for developing strictures and with an early onset of disease. [10] In the future, these variants may be helpful in predicting the course of the disease in affected individuals.
Patient Education
Education of patients and their families is encouraged and is extremely important in the treatment process. Useful education materials can be obtained from the following organization:
-
Crohn’s and Colitis Foundation of America, 386 Park Avenue South, 17th floor, New York, NY 10016; (800) 932-2423; http://www.ccfa.org; e-mail: info@ccfa.org
In addition, see the Digestive Disorders Center, as well as Inflammatory Bowel Disease, Crohn’s Disease, Crohn’s Disease FAQs, and Ulcerative Colitis.
-
Crohn disease. The colonoscopy image reveals a large ulcer and inflammation of the descending colon in a 12-year-old boy with Crohn disease.
-
Crohn disease. This laparoscopic view depicts creeping fat along the mesentery of the terminal ileum.
-
Crohn disease. On this laparoscopic photograph, the mesentery of the terminal ileum is being coagulated with a sealing device (LigaSure). Note that the ligation of the mesentery proceeds near the border of the ileum rather than at the base of the mesentery.
-
Crohn disease. This postoperative photograph depicts incisions used for laparoscopic ileocolectomy in a 14-year-old male adolescent with obstruction of the terminal ileum. Note the 2-cm incision in the right lower abdomen, through which the specimen was extracted and extracorporeal anastomosis performed. A 12-mm umbilical incision is nicely hidden in the depths of the umbilicus. A 5-mm incision is visible in the left lower abdomen, and another is in the left suprapubic region just above the top of the pants.
-
Crohn disease. Colonic granuloma in a patient with Crohn disease is shown (hematoxylin-eosin staining). Image courtesy of Dr E. Ruchelli.
-
Crohn disease. Aphthous ulcers. A double-contrast barium enema examination in a patient with Crohn colitis demonstrates numerous aphthous ulcers.
-
Crohn disease. This double-contrast barium enema study demonstrates marked ulceration, inflammatory changes, and narrowing of the right colon in a patient with Crohn colitis.
-
Crohn disease. Cobblestoning in Crohn disease. This is a spot view of the terminal ileum from a small bowel follow-through study. It demonstrates linear longitudinal and transverse ulcerations that create a cobblestone appearance. Also, note the relatively greater involvement of the mesenteric side of the terminal ileum and the displacement of the involved loop away from the normal small bowel secondary to mesenteric inflammation and fibrofatty proliferation.
-
Crohn disease. Crohn disease of terminal ileum. The small bowel follow-through study demonstrates the string sign in the terminal ileum. Also, note the pseudodiverticula of the antimesenteric wall of the terminal ileum, secondary to greater distensibility of this less-involved wall segment.
-
Crohn disease. This spot view of the terminal ileum from a small bowel follow-through study in a patient with Crohn disease demonstrates the string sign, consistent with narrowing and stricturing. Also, note a sinus tract originating from the medial wall of the terminal ileum and the involvement of the medial wall of the cecum.
-
Crohn disease. Enterocolic fistula in a patient with Crohn disease. The double-contrast barium enema study demonstrates multiple fistulous tracts between the terminal ileum and the right colon adjacent to the ileocecal valve (so-called double-tracking of the ileocecal valve).
-
Crohn disease. Active small bowel inflammation in a patient with Crohn disease. This computed tomography scan demonstrates small bowel wall thickening, mesenteric inflammatory stranding, and mesenteric adenopathy.
-
Crohn disease. This computed tomography scan from a patient with terminal ileal Crohn disease shows an enteroenteral fistula (arrow) between loops of diseased small intestine.
-
Crohn disease. A teenaged patient with Crohn disease underwent contrast-enhanced, upper gastrointestinal computed tomography scanning with small-bowel follow-through. Several loops of small bowel are in the pelvis. Note the loop of distal bowel with thickened wall (solid arrow), which is contrasted with a less-involved loop of bowel in which the intestinal wall is not thickened at all (dotted arrow).
-
Crohn disease. This computed tomography scan depicts Crohn disease in the fundus of the stomach.
-
Crohn disease. This magnetic resonance image demonstrates an inflamed terminal ileum in 10-year-old girl with Crohn disease.
-
Crohn disease. The histologic image shows granuloma in the mucosa of a patient with Crohn disease.