Background
Corynebacteria (from the Greek words koryne, meaning club, and bacterion, meaning little rod) are gram-positive, catalase-positive, aerobic or facultatively anaerobic, generally nonmotile rods. The genus contains the species Corynebacterium diphtheriae and the nondiphtherial corynebacteria, collectively referred to as diphtheroids. Nondiphtherial corynebacteria, originally thought to be mainly contaminants, have increasingly over the past 2 decades been recognized as pathogenic, especially in immunocompromised hosts.
Approximately 30 years ago, taxonomic changes were made to diverse genera previously included within the coryneform groups. The reclassification is based on the degree of homology of RNA oligonucleotides between groups. Based on this reclassification, for example, Corynebacterium haemolyticum became Arcanobacterium haemolyticum and the JK group became Corynebacterium jeikeium. [1] More recently, Van den Velde and colleagues have suggested that species of corynebacteria would be more correctly identified based on their cellular fatty acid profiles (ie, for the C14 to C20 fatty acids). [2]
Advances in molecular biology and genome analysis now also allow for detailed descriptions of DNA-binding transcription factors and transcriptional regulatory networks. This was first described for Corynebacterium glutamicun. Web-based resources are available online at CoryneRegNet [3] and CoryneCenter. [4]
Prior to the 1990s, the incidence of diphtheria had been declining. However, an epidemic of diphtheria in the former Soviet Union was first noticed in the Russian Republic in 1990 and then spread to the other newly independent states, peaking in the mid-1990s. In some endemic locations, such as India, 44% of throat and nasal swabs tested positive for C diphtheriae and Corynebacterium pseudodiphtheriticum. [5] Today, the more common scenario is nondiphtherial corynebacterial bacteremia associated with device infections (venous access catheters, heart valves, neurosurgical shunts, peritoneal catheters), as well as meningitis, septic arthritis, and urinary tract infections.
For more information about C diphtheriae infections, please see Diphtheria.
Most recently, an increase in nondiphtherial corynebacterial infections of the skin and soft tissues has been reported. [6, 7, 8, 9, 10, 11, 12, 13, 14]
Nondiphtherial corynebacteria also cause chronic and subclinical diseases in domestic animals and can lead to significant economic losses for farmers. Examples of widespread and difficult-to-control infections include Corynebacterium pseudotuberculosis caseous lymphadenitis in sheep, goats, and alpacas; C pseudotuberculosis ulcerative dermatitis in cattle; and urinary tract infections and mastitis (affecting milk production) in cattle due to infection with Corynebacterium renale, Corynebacterium cystidis, Corynebacterium pilosum, and Corynebacterium bovis. [15, 16]
Pathophysiology
C diphtheriae
C diphtheriae infection typically is characterized by a local inflammation, usually in the upper respiratory tract, associated with toxin-mediated cardiac and neural disease. Three strains of C diphtheriae are recognized, in decreasing order of virulence: gravis, intermedius, and mitis. These strains all produce an identical toxin, but the gravis strain is potentially more virulent because it grows faster and depletes the local iron supply, allowing for earlier and greater toxin production. Toxin production is encoded on the tox gene, which, in turn, is carried on a lysogenic beta phage. When DNA of the phage integrates into the host bacteria's genetic material, the bacteria develop the capacity to produce this polypeptide toxin.
The tox gene is regulated by a corynebacterial iron-binding repressor (DtxR). In the presence of ferrous iron, the DtxR-iron complex attaches to the tox gene operon, inhibiting transcription. In an iron-poor environment, the DtxR molecule is released and the tox gene is transcribed (see the illustration below).
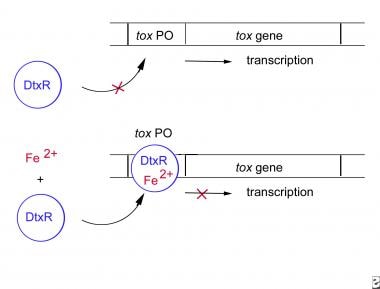 The corynebacterial tox gene is regulated by the corynebacterial iron-binding repressor, labeled DtxR. Binding of ferrous iron to the DtxR molecule forms a complex that binds to the tox gene operator and inhibits transcription. Depletion of iron from the system removes the repression and allows the toxin to be produced.
The corynebacterial tox gene is regulated by the corynebacterial iron-binding repressor, labeled DtxR. Binding of ferrous iron to the DtxR molecule forms a complex that binds to the tox gene operator and inhibits transcription. Depletion of iron from the system removes the repression and allows the toxin to be produced.
The toxin is a single polypeptide with an active (A) domain, a binding (B) domain, and a hydrophobic segment known as the T domain, which helps release the active part of the polypeptide into the cytoplasm. In the cytosol, the A domain catalyzes the transfer of an adenosine diphosphate-ribose molecule to one of the elongation factors (eg, elongation factor 2 [EF2]) responsible for protein synthesis. This transfer inactivates the factor, thereby inhibiting cellular protein synthesis. Inhibiting all the protein synthesis in the cell causes cell death.
In this manner, the toxin is responsible for many of the clinical manifestations of the disease. As little as 0.1 µg can cause death in guinea pigs. In 1890, von Behring and Kitasato demonstrated that sublethal doses of the toxin induced neutralizing antibodies against the toxin in horses. In turn, this antiserum passively protected the animals against death following challenge infection. By the early 1900s, treating the toxin with heat and formalin was discovered to render it nontoxic. When injected into recipients, the treated toxin induced neutralizing antibodies. By the 1930s, many Western countries began immunization programs using this toxoid.
Adhesion of pathogenic corynebacteria to host cells is a crucial step during infection. Adhesion to host cells is mediated by filaments called fimbrae or pili; the minor pilins SpaB and SpaC are specific adhesins that covalently bind the bacteria to the cell membranes of the respiratory epithelium. [17]
More recently, iron levels have been shown to regulate the adhesion properties of the bacteria; iron-limited conditions promote changes in the cell-surface residues, leading to increased hemagglutination activity and decreased binding to glass. [18]
The disease occurs mainly in temperate zones and is endemic in certain regions of the world. Most US cases are sporadic or occur in nonimmunized persons. Humans are the only known reservoir for the disease. The primary modes of dissemination are by airborne respiratory droplets, direct contact with droplets, or infected skin lesions. Asymptomatic respiratory carrier states are believed to be important in perpetuating both endemic and epidemic disease. Immunization reduces the likelihood of carrier status.
Bacteria usually gain entry to the body through the upper respiratory tract, but entry through the skin, genital tract, or eye is also possible. The cell surface of C diphtheriae has three distinct pilus structures: the main pilus shaft (SpaA) and two small pili (SpaB, SpaC). Adherence to respiratory epithelial cells can be greatly diminished by blocking production of these two minor pili or by using antibodies directed against them. [19]
In most cases, C diphtheriae infection grows locally and elicits toxin rather than spreading hematogenously. The characteristic membrane of diphtheria is thick, leathery, grayish-blue or white and composed of bacteria, necrotic epithelium, macrophages, and fibrin. The membrane firmly adheres to the underlying mucosa; forceful removal of this membrane causes bleeding. The membrane can spread down the bronchial tree, causing respiratory tract obstruction and dyspnea.
The toxin-induced manifestations involve mainly the heart, kidneys, and peripheral nerves. Cardiac enlargement due to myocarditis is common. The kidneys become edematous and develop interstitial changes. Both the motor and sensory fibers of the peripheral nerves demonstrate fatty degenerative changes and disintegration of the medullary sheaths. The anterior horn cells and posterior columns of the spinal canal can be involved, and the CNS may develop signs of hemorrhage, meningitis, and encephalitis. Death is mainly due to respiratory obstruction by the membrane or toxic effects in the heart or nervous system.
In recent years, the epidemiology of C diphtheriae infection has been changing. Increasing numbers of skin, pharyngeal, and bacteremic infections with nontoxigenic bacteria have been reported. Among 828 cultures of nontoxigenic C diphtheriae isolated from different regions of Russia from 1994-2002, 14% carried the gene for the toxin. [20] Molecular characterization based on polymerase chain reaction (PCR) of some of these nontoxigenic strains have demonstrated that the bacteria often contain functional DtxR proteins, which could potentially produce toxin. [21]
Other corynebacteria (ie, diphtheroids)
Nondiphtherial corynebacteria are ubiquitous in nature and commonly colonize human skin and mucous membranes. Only recently has the role of these organisms in human infections been appreciated. In the past, many of these organisms cannot be speciated or typed easily, even in research laboratories; now recent advances in PCR technology are improving our ability to identify these bacteria. Coyle and Lipsky in 1990 reviewed some of the common coryneform bacteria that cause infections. [1]
Specific pathogenic groups or species include the following:
-
Corynebacterium ulcerans
-
C pseudotuberculosis (also known as Corynebacterium ovis)
-
Corynebacterium pyogenes
-
A haemolyticum
-
Corynebacterium aquaticum
-
C pseudodiphtheriticum (also known as Corynebacterium hofmannii)
-
Group D2 (also known as Corynebacterium urealyticum)
-
Group E
-
C jeikeium (ie, group JK)
Since then, more than 80 species have been described, [22] of which two thirds are either pathogenic in animals, especially in livestock; or can be transmitted to humans by zoonotic contact. Depending on the species, both skin and internal-organ systems can be affected, particularly in patients who are elderly, are immunosuppressed, or have multiorgan dysfunction. Recent genomic analyses of diphtheroids demonstrated diversified genomic island containing genes for virulence and multidrug resistance. [11] This helps explain why some (eg, group D2) are highly resistant and require susceptibility testing for optimal treatment.
Epidemiology
Frequency
United States
C diphtheriae
In immunized persons, the rate of C diphtheriae infection since 1980 has been extremely low (< 5 cases per 100,000 population). Although infection can occur in immunized persons, prior immunization decreases disease frequency and severity. However, disease incidence started to fall even before the widespread use of toxoid. This decline may have been due to a decreasing incidence of bacterial carriers. In addition, immunized persons are less likely to be carriers of toxigenic phages.
Persons who have never been immunized or those incompletely immunized or with waxing immunity are at an increased risk for infection. In the United States, this group mainly consists of poorer individuals and immigrants.
Diphtheroids
Infections with nondiphtherial corynebacteria are being reported more frequently, especially those associated with medical devices such as intravascular catheters, artificial valves, and CNS drainage devices.
Moazzez et al (2007) found that 16% of breast abscesses in an urban county hospital were due to diphtheroids. [23]
International
C diphtheriae infection: In the early 1990s, the World Health Organization (WHO) reported that diphtheria was still endemic in many parts of the world (eg, Brazil, Nigeria, the Indian subcontinent, Indonesia, Philippines, some parts of the former Soviet Union [especially St. Petersburg and Moscow]), with epidemics also reported in republics of the former Soviet Union. The February 2000 supplement (vol.181) of the Journal of Infectious Diseases contains an in-depth evaluation of the epidemic. [24]
The Kyrgyz Republic experienced a widespread resurgence of diphtheria from 1994-1998. Among 676 patients hospitalized with respiratory diphtheria, 163 (24%) were carriers, 186 (28%) had tonsillar forms, 78 (12%) had combined types or delayed diagnosis, and 201 (30%) had severe forms. The highest age-specific incidence rates occurred among persons aged 15 years to 34 years; 70% of cases were among those aged 15 years or older. Myocarditis occurred among 151 patients (22%), and 19 patients died (case fatality rate of 3%). [25]
In another epidemic in the Republic of Georgia from 1993-1996, 659 cases and 68 deaths were reported (case fatality rate of 10%). More than 50% of the cases and deaths were in children aged 14 years and younger (case fatality rate of 16%) and in adults aged 40 years to 49 years (case fatality rate of 19%). [26]
During 2007-2008, 10 European countries screened patients with respiratory infections with throat swabs; carriage rates for nontoxigenic Corynebacterium organisms ranged from 0 to 4 cases per 1000 patients. [27]
Sporadic C diphtheriae infections are reported annually. These include skin and bloodstream infections. A review of 85 isolates from the United Kingdom from 1998-2003 revealed that most the reports came from one hospital in London, suggesting that the true incidence may be higher. [28]
Another recent review of C diphtheriae infections in Brazil and South America emphasized a shift in biotype, with an increase in the dissemination of an atypical sucrose-fermenting biotype, which appears to have an enhanced ability to colonize and to spread. [29]
In New Zealand, C diphtheria infections were associated with infective endocarditis in children in 12% of cases (10 of 85 cases) from 1994-2012. [30]
Diphtheroids
Infections with the nondiphtherial corynebacteria are reported worldwide. [31] Some (eg, group D2) originally reported in Europe are now found in the United States, while the JK group initially reported in the United States is found in Europe.
Egwari et al (2008) found that, in east Africa, 9.7% of odontogenic infections that progressed to sepsis were due to diphtheroids. [32]
The incidence of C ulcerans infections in the United Kingdom associated with contact with exposed animals has increased over the past 2 decades, becoming more common than C diphtheriae infections. [33]
C ulcerans has also been reported in Latin America and one fatal case was noted in Brazil in 2008, in an elderly woman with disseminated disease resistant to penicillin and clindamycin. [34]
Mortality/Morbidity
C diphtheriae
Mortality rates are highest at the extremes of age and in insufficiently immunized persons. However, even partial immunization confers a reduced risk of severe disease. Death usually occurs within the first week, either from asphyxia or heart disease.
Immunity to diphtheria waxes in the absence of booster injections of toxoid or natural infection. Therefore, persons traveling to endemic areas should receive booster injections. At one time, diphtheria immunization was considered lapsed if more than 4 years had elapsed since the last booster. This estimate is probably still relevant for persons traveling to high-risk areas, particularly those in high-risk jobs, such as medical personnel. Otherwise, the routine recommendation is currently for booster injections every 10 years. Annual updates are made each year by the CDC. A complete Adult Immunization Schedule is available from the CDC's National Immunization Program.
Diphtheroids
These infections tend to occur in patients who are elderly, neutropenic, or immunocompromised or who have prosthetic devices (eg, heart valves, dialysis catheters, neurologic shunts).
Race
C diphtheriae
The respiratory form of this disease has no racial predilection. Since 1972, the prevalence of the cutaneous form of the disease has increased in the United States, with a high attack rate among Native Americans and in indigent areas where crowding and poor personal and community hygiene are common. Three outbreaks of C diphtheriae infection, 86% of which were cutaneous, were recorded in Seattle's Skid Road from 1972-1982. [35]
Diphtheroids
No racial predilection exists.
Sex
No sexual predilection is reported for any of the corynebacterial diseases.
Age
C diphtheriae
The incidence of infection in children who are not immunized is reported as 70 times higher than in children who have received primary immunization. In the recent epidemics in the republics of the former Soviet Union, the high rate of infection among adults aged 40 years to 49 years has been attributed to their low levels of immunity.
Diphtheroids
Infections are reported in children and elderly persons.
Patient Education
Vaccination is the key to preventing C diphtheriae infections. Public health services and individual physicians are important resources for providing appropriate treatments. Vaccination is especially important for high-risk groups (eg, children, elderly individuals, immigrants from areas of continued endemic infections).
Infections with other diphtheroids are becoming an increasingly important problem in immunocompromised individuals; updated education of physicians caring for these patients is needed.
For patient education resources, see the Children's Health Center and Public Health Center, as well as Immunization Schedule, Children and Immunization Schedule, Adults.
-
The corynebacterial tox gene is regulated by the corynebacterial iron-binding repressor, labeled DtxR. Binding of ferrous iron to the DtxR molecule forms a complex that binds to the tox gene operator and inhibits transcription. Depletion of iron from the system removes the repression and allows the toxin to be produced.
-
The characteristic thick membrane of diphtheria infection in the posterior pharynx.
-
Cervical edema and cervical lymphadenopathy from diphtheria infection produce a bullneck appearance in this child. Courtesy of Immunize.org, formerly Immunization Action Coalition (IAC) [https://www.immunize.org/].
-
Final case classification of corynebacteria infections. Courtesy of the World Health Organization (WHO) [Surveillance standards for vaccine-preventable diseases, 2nd ed, 2018, https://apps.who.int/iris/handle/10665/275754].






