Practice Essentials
Colon cancer is the most common type of gastrointestinal cancer. It is a multifactorial disease process, with etiology encompassing genetic factors, environmental exposures (including diet), and inflammatory conditions of the digestive tract.
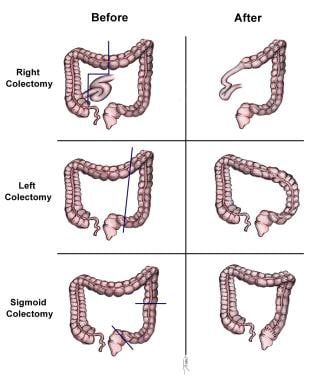
Surgery currently is the definitive treatment modality. [1] The image below depicts standard colectomies for adenocarcinoma of the colon.
Signs and symptoms
Colon cancer is now often detected during screening procedures. Common clinical presentations include the following:
-
Iron-deficiency anemia
-
Rectal bleeding
-
Abdominal pain
-
Change in bowel habits
-
Intestinal obstruction or perforation
Physical findings may include the following:
-
Early disease: Nonspecific findings (fatigue, weight loss) or none at all
-
More advanced disease: Abdominal tenderness, macroscopic rectal bleeding, palpable abdominal mass, hepatomegaly, ascites
See Presentation for more detail.
Diagnosis
Laboratory studies that may be helpful include the following:
-
Complete blood count
-
Chemistries and liver function tests
-
Serum carcinoembryonic antigen
Imaging studies that may facilitate staging include the following:
-
Chest radiography
-
Chest computed tomography
-
Abdominal barium study
-
Abdominal/pelvic CT
-
Contrast ultrasonography of the abdomen and liver
-
Abdominal/pelvic MRI
-
Positron emission tomography, including fusion PET-CT scan
Other procedures that may be warranted include the following:
-
Colonoscopy
-
Sigmoidoscopy
-
Biopsy of suspicious lesions
-
Double-contrast barium enema
See Workup for more detail.
Management
Surgery is the only curative modality for localized colon cancer (stage I-III). Surgical resection potentially provides the only curative option for patients with limited metastatic disease in liver and/or lung (stage IV disease). Surgical options include the following:
-
Right hemicolectomy: For lesions in the cecum and right colon
-
Extended right hemicolectomy: For lesions in the proximal or middle transverse colon
-
Left hemicolectomy: For lesions in the splenic flexure and left colon
-
Sigmoid colectomy: For sigmoid colon lesions
-
Total abdominal colectomy with ileorectal anastomosis: For selected patients with hereditary nonpolyposis colon cancer, attenuated familial adenomatous polyposis, metachronous cancers in separate colon segments, or acute malignant colon obstructions with unknown status of the proximal bowel
Other therapeutic options for patients who are not surgical candidates include the following:
-
Cryotherapy
-
Radiofrequency ablation
-
Hepatic arterial infusion of chemotherapeutic agents
Adjuvant (postoperative) therapy is used in selected patients with stage II colon cancer who are at high risk of recurrence, and is standard for stage III colon cancer. Regimens used for systemic chemotherapy may include the following:
-
5-Fluorouracil (5-FU)
-
Capecitabine
-
Oxaliplatin
-
Combinations of multiple agents (eg, capecitabine or 5-FU with oxaliplatin, FOLFOX, FOLFIRI, cetuximab or panitumumab with encorafenib)
Regimens used for adjuvant chemotherapy commonly include 5-FU with leucovorin or capecitabine, either alone or in combination with oxaliplatin. [2, 3, 4]
For metastatic colon cancer, systemic chemotherapy is standard, with neoadjuvant chemotherapy used to convert unresectable isolated liver metastases to resectable liver metastases. Biologic agents have assumed a major role, typically as targeted therapy based on genetic analysis of the tumor. Biologic agents employed to treat colon cancer include the following:
-
Bevacizumab (Avastin)
-
Cetuximab (Erbitux)
-
Ipilimumab (Yervoy)
-
Nivolumab (Opdivo)
-
Panitumumab (Vectibix)
-
Pembrolizumab (Keytruda)
-
Ramucirumab (Cyramza)
-
Tucatinib (Tukysa)
-
Fruquintinib (Fruzaqla)
See Treatment and Medication for more detail.
For more information, see Colorectal Cancer Guidelines.
Go to Oncology Decision Point Colorectal Cancer for expert commentary on treatment decisions and related guidelines.
For patient education information, see Colon Cancer.
Background
Invasive colorectal cancer is a preventable disease. Early detection through widely applied screening programs is the most important factor in the recent decline of colorectal cancer in developed countries (see Overview/Epidemiology).
Fundamental advances in understanding the biology and genetics of colorectal cancer are taking place. This knowledge is slowly making its way into the clinic and being employed to better stratify individual risks of developing colorectal cancer, discover better screening methodologies, allow for better prognostication, and improve the ability to predict benefit from new anticancer therapies.
In the past 10 years, an unprecedented advance in systemic therapy for colorectal cancer has dramatically improved outcome for patients with metastatic disease. Until the mid-1990s, the only approved agent for colorectal cancer was 5-fluorouracil. Since then, new agents in a variety of classes have become available, including the following:
-
Cytotoxic agents (eg, irinotecan, oxaliplatin) [5]
-
Oral fluoropyrimidines (ie, capecitabine)
-
Biologic agents (eg, bevacizumab, cetuximab, panitumumab, pembrolizumab, nivolumab) [1]
-
Most recently, anti-angiogenic agents (ie, ziv-aflibercept, regorafenib)
Although surgery remains the definitive treatment modality, these new agents will likely translate into improved cure rates for patients with early-stage disease (stage II and III) and prolonged survival for those with stage IV disease. Further advances are likely to come from the development of new targeted agents and from better integration of systemic therapy with other modalities such as surgery, radiation therapy, and liver-directed therapies.
Pathophysiology
Genetically, colorectal cancer represents a complex disease, and genetic alterations are often associated with progression from premalignant lesion (adenoma) to invasive adenocarcinoma. Sequence of molecular and genetic events leading to transformation from adenomatous polyps to overt malignancy has been characterized by Vogelstein and Fearon. [6]
The early event is a mutation of APC (adenomatous polyposis gene), which was first discovered in individuals with familial adenomatous polyposis (FAP). The protein encoded by APC is important in the activation of oncogene c-myc and cyclin D1, which drives the progression to malignant phenotype. Although FAP is a rare hereditary syndrome accounting for only about 1% of cases of colon cancer, APC mutations are very frequent in sporadic colorectal cancers.
Other important genes in colon carcinogenesis include the KRAS oncogene, chromosome 18 loss of heterozygosity (LOH) leading to inactivation of SMAD4 (DPC4), and DCC (deleted in colon cancer) tumor suppression genes. Chromosome arm 17p deletion and mutations affecting the p53 tumor suppressor gene confer resistance to programmed cell deat—h (apoptosis) and are thought to be late events in colon carcinogenesis.
A subset of colorectal cancers is characterized with deficient DNA mismatch repair. This phenotype has been linked to mutations of genes such as MSH2, MLH1, and PMS2. These mutations result in so-called high frequency microsatellite instability (H-MSI), which can be detected with an immunocytochemistry assay. H-MSI is a hallmark of hereditary nonpolyposis colon cancer syndrome (HNPCC, Lynch syndrome), which accounts for about 6% of all colon cancers. H-MSI is also found in about 20% of sporadic colon cancers.
In addition to mutations, epigenetic events such as abnormal DNA methylation can also cause silencing of tumor suppressor genes or activation of oncogenes. These events compromise the genetic balance and ultimately lead to malignant transformation.
Cancer cells produce extracellular vesicles (EVs)—principally, microvesicles and exosomes—that can promote the growth, survival, invasiveness, and metastatic activity of tumors. [7] Zhao et al reported that in an animal model of aggressive late-stage colorectal cancer, tumor-secreted EVs promoted resistance to immune checkpoint blockade. The colorectal cancer cells in this model secrete exosomes that carry immunosuppressive microRNAs these block CD28 on T cells and CD80 on dendritic cells that infiltrate the tumors, disabling T-cell–mediated anti-tumor immune response. [8]
Further, these authors found that intravenous injections of tumor-secreted exosomes without immunosuppressive microRNAs, in combination with immune checkpoint inhibitors, resulted in an enhanced anti-tumor immune response. This offers a potential therapeutic strategy for late-stage colorectal cancer. [8]
Etiology
Colorectal cancer is a multifactorial disease process. Genetic factors, environmental exposures (including diet), and inflammatory conditions of digestive tract are all involved in the development of colorectal cancer.
Although much about colorectal cancer genetics remains unknown, current research indicates that genetic factors have the greatest correlation to colorectal cancer. Hereditary mutation of the APC gene is the cause of familial adenomatous polyposis (FAP), in which affected individuals carry an almost 100% risk of developing colon cancer by age 40 years.
Hereditary nonpolyposis colon cancer syndrome (HNPCC, Lynch syndrome) poses about a 40% lifetime risk for developing colorectal cancer; individuals with this syndrome are also at increased risk for urothelial cancer, endometrial cancer, and other less common cancers. Lynch syndrome is characterized by deficient mismatch repair (dMMR) due to inherited mutation in one of the mismatch repair genes, such as hMLH1, hMSH2, hMSH6, hPMS1, hPMS2, and possibly other undiscovered genes.
HNPCC is a cause of about 6% of all colon cancers. Although the use of aspirin may reduce the risk of colorectal neoplasia in some populations, a study by Burn et al found no effect on the incidence of colorectal cancer in carriers of Lynch syndrome with use of aspirin, resistant starch, or both. [9]
Dietary factors are the subject of intense and ongoing investigations. [10] Epidemiologic studies have linked increased risk of colorectal cancer with a diet high in red meat and animal fat, low-fiber diets, and low overall intake of fruits and vegetables. A study by Aune et al found that a high intake of fiber was associated with a reduced risk of colorectal cancer. In particular, cereal fiber and whole grains were found to be effective. [11] A study by Pala et al found that high yogurt intake was also associated with a decreased risk for colorectal cancer. [12]
A cohort study by Tabung et al that followed 121,050 adults for 26 years found that in both men and women, intake of proinflammatory diets (replete in red, processed, and organ meat, for example) was associated with a significantly higher risk of developing colorectal cancer. Risk was especially high in overweight and obese men and, paradoxically, in lean women. Risk was also increased in men and women who do not drink alcohol. [13]
Factors associated with lower risk include folate intake, calcium intake, and estrogen replacement therapy. However, most of these studies were retrospective epidemiologic studies and have yet to be validated in prospective, placebo-controlled, interventional trials.
Obesity and lifestyle choices such as cigarette smoking, alcohol consumption, and sedentary habits have also been associated with increased risk for colorectal cancer. A meta-analysis of prospective cohort studies found a modest but significant elevation of colorectal cancer risk in current smokers; risk was higher for men and for rectal cancers than colon cancers, and persisting in former smokers. [14]
In a large prospective study, Cho and colleagues reported that high alcohol consumption was associated with elevated risk for colorectal cancer, in individuals with a family history of the disease. The association was significant only for the highest alcohol intake category of 30 g or more daily; no significant linear trend was evident. In comparison with nondrinkers with no family history, individuals who consumed 30 g/d or more and who had a family history of colorectal cancer had a relative risk for colon cancer of 2.80. [15]
Current screening guidelines recommend that clinicians be aware of increased colorectal cancer risk in patients who smoke or are obese, but do not highlight the increased risk in patients with diabetes. A meta-analysis of case-control and cohort studies identified diabetes as an independent risk factor for colon and rectal cancer. Subgroup analyses confirmed the consistency of the findings across study type and population. This information may have an impact on screening guidelines and on building risk models of colorectal cancer. [16]
Association between body mass index (BMI) and risk of colorectal adenomas and cancer has been reported, but few studies have had adequate sample size for conducting stratified analyses. Jacobs et al pooled data from 8,213 participants in seven prospective studies and found that BMI was significantly related to most histologic characteristics of metachronous adenomas in men but not in women. The researchers concluded that body size may affect colorectal carcinogenesis at comparatively early stages, particularly in men. [17]
A nationwide cohort study from France of incident colorectal cancer in obese patients, which compared outcomes in 74,131 patients who underwent bariatric surgery with 971, 217 patients who did not have surgery, found that in the bariatric surgery cohort, risk of colorectal cancer was the same as that in the general population. In the obese patients who did not undergo bariatric surgery, the risk was 34% above that of the general population. [18]
Activation of the WNT signaling pathway, which most often results from APC loss, plays a critical role in the development of colorectal cancer, and CTNNB1 (β-catenin) is a major mediator of the WNT pathway. WNT-CTNNB1 signaling also appears to be involved in obesity, glucose metabolism, and metabolic diseases such as obesity and type II diabetes. Consequently, Morikawa et al hypothesized that the association of obesity and physical activity with colorectal cancer risk might differ by tumor subtypes according to CTNNB1 status. [19]
Using a molecular pathological epidemiology database, these researchers determined that risk of CTNNB1-negative cancer was significantly higher with greater BMI and lower with increased physical activity level. These researchers found no association between either BMI or physical activity level and CTNNB1-positive cancer risk. [19]
Greater adult-attained height is associated with an increased risk of colorectal cancer and adenoma, according to a systematic review and meta-analysis by Zhou et al that included 47 observational studies involving 280,644 colorectal cancer and 14,139 colorectal adenoma cases.The study found that overall, the risk of colorectal cancer is 24% higher in the tallest individuals within the highest percentile of height, compared with the shortest individuals within the lowest percentile. Every 3.9-inch (10-centimeter) increase in height was associated with a 14% higher risk for colon cancer and 6% higher odds of adenoma. Zhou et al recommend considering height as a risk factor for colorectal cancer screening. [20]
Excessive consumption of beverages sweetened with high-fructose corn syrup (HFCS) is associated with increased risk of colorectal cancer. In a study of adenomatous polyposis coli (APC) mutant mice, which are predisposed to develop intestinal tumors, daily administration of 20 g of weight-adjusted HFCS (the equivalent of 1 soda a day) resulted in a substantial increase in in polyps that rapidly developed into advanced, high-grade dysplastic lesions. Carbon labeling showed uptake in fructose within the intestinal tumors themselves. Within the tumors, fructose was converted to fructose-1-phosphate, leading to activation of glycolysis and increased synthesis of fatty acids that support tumor growth. [21]
Inflammatory bowel diseases such as ulcerative colitis and Crohn disease also increase the risk of developing colorectal adenocarcinoma. The risk for developing colorectal malignancy increases with the duration of inflammatory bowel disease and the greater extent of colon involvement.
A matched case-control study of incident colorectal cancer cases in the United Kingdom from 1989 to 2012 found that use of oral antibiotics was associated with increased risk of colon cancer, particularly in the proximal colon. The association involved antibiotic exposure occurring more than 10 years before colon cancer diagnosis. Risk was dose dependent but was observed after even a single course of antibiotics. In addition, risk was greatest with anti-anaerobic antibiotics. The authors note that such antibiotics markedly disrupt the gut microbiome, which consists predominantly of anaerobes, and this disruption may facilitate the acquisition or development of a carcinogenic colon microbiota. [22]
Epidemiology
The incidence and mortality from colon cancer have been on a slow decline over the past several decades in the United States, with the incidence falling on average 2.4% each year and death rates falling on average 2.2% each year over 2007-2019. [23] However, colorectal cancers remain the third most common cancer and third most common cause of cancer-related mortality in US men and women. [24] In addition, rates of colon cancer in younger persons have been increasing. [25]
The American Cancer Society estimates that 106,970 new cases of colon cancer will be diagnosed in the United States in 2023. Estimates for mortality from colon and rectal cancer (the two are combined because of classification difficulties) are for 52,550 deaths in 2023. [24]
A case-control study using national Veterans Affairs–Medicare data concluded that colonoscopy was associated with significant reductions in colorectal cancer mortality in veterans. Mortality benefit was greater for left-sided cancer than right-sided cancer. [26] Case patients (n= 4964) were veterans aged 52 years or older who were diagnosed with colorectal cancer in 2002 to 2008 and died of the disease by the end of 2010. Case patients were matched to four control patients (n= 9,856) without prior colorectal cancer. Risk of mortality from left-sided cancer was reduced in those who had undergone colonoscopy (odds ratio [OR], 0.28 [CI, 0.24 to 0.32]), as was risk for mortality from right-sided cancer (OR, 0.54 [CI, 0.47 to 0.63]). [26]
Worldwide, an estimated 1,931,590 new cases of colorectal cancer occurred in 2020 (10% of all cancers). Geographically, the incidence varies as much as six-fold. The highest estimated rates are in southern Europe (per 100,000 population, 40.6 in men and 24.5 in women), and the lowest in south-central Asia (per 100,000 population, 6.6 in men and 4.4 in women). [27]
Worldwide, colorectal cancer caused approximately 935,173 deaths in 2020, accounting for 9.4% of cancer mortality overall. As with incidence rates, mortality rates worldwide vary six-fold, with the highest estimated mortality rates in central and eastern Europe (14.5 per 100,000 population), and the lowest in south-central Asia (3.2 per 100,000 population). [27]
An epidemiologic study from the European Union (EU) concluded that in 2018, colorectal cancer would account for the second highest number of cancer deaths, at 98,000 deaths in men and 79,400 in women. However, while the total number of colorectal deaths in the EU has risen since 2012 because of the aging population, since 2012 the age-standardized death rate has fallen by 6.7% (to 15.8 per 100,000 in men and 7.5% (to 9.2 per 100,000) in women. [28]
Racial, sexual, and age-related disparities in incidence
Since 1989, colorectal cancer incidence rates have been higher for Blacks than for Whites in both men and women. Currently, incidence rates of colorectal cancer are 21% higher in Black men and 18% higher in Black women compared with White men and women, respectively. [29]
Colorectal mortality rates are 44% higher in Black men and 31% higher in Black women compared with White men and women. However, from 2010 to 2019, colorectal cancer death rates declined faster in Blacks than in Whites (2.8% vs 1.8% per year), narrowing the racial disparity in both men and women. [29]
Asians/Pacific Islanders have the lowest incidence and mortality from colorectal cancer. Hispanics have the second lowest. [23]
The incidence of colorectal cancer is relatively equal in men and women. The American Cancer Society estimates that colon cancer will be diagnosed in 54,420 men and 52,550 women in the United States in 2023. [24]
Age is a well-known risk factor for colorectal cancer, as it is for many other solid tumors. The timeline for progression from early premalignant lesion to malignant cancer ranges from 10-20 years. Median age at diagnosis is 66 years. [23]
However, in contrast to the decline in colon cancer incidence rates in persons age 55 and older, which began in the mid-1980s, rates of colon cancer in younger persons have been increasing. In adults age 20 to 39 years, colon cancer incidence rates have increased by 1.0% to 2.4% annually since the mid-1980s; in those age 40 to 54 years, the incidence has increased by 0.5% to 1.3% annually since the mid-1990s. Currently, adults born circa 1990 have double the risk of colon cancer compared with those born circa 1950. Increased obesity is one likely factor. [25]
From 2011 through 2016, the incidence of colorectal cancer continued to decline in those aged 65 years and older, by 3.3% annually. Rates increased by 1% annually in those aged 50 to 64 years, and rose approximately 2% annually in those younger than 50 years. The American Cancer Society estimated that 17,930 of the 147,950 individuals expected to be diagnosed with colon and rectal cancer in 2020, and 3640 of the 53,200 expected to die from the disease, would be younger than 50 years of age. [30]
Tumor site tends to vary by patient age. From 2012 to 2016, the proximal colon was the site of colon cancer in 23% of those under 50 years of age, 31% of those 50-64 years, and 49% of those 65 and older. Incidence trends varied by race/ethnicity: in those 50-64 years old, rates increased in whites by 1.3% per year but decreased in blacks by 1.6% per year, and were stable in Hispanics. In those younger than 50, rates rose by 2% annually in whites and by 0.5% annually in Blacks. [30]
A review of Surveillance, Epidemiology and End Results (SEER) data found that US cases of colorectal cancer in persons aged 40-49 years have increased significantly since 1995, with the greatest average annual percentage increase for distant cancers, at 2.9%, while localized and regional disease each increased < 1.5% per year. In addition, the proportion of distant colorectal cancers in this age group increased significantly from 1990-1994 to 2011–2015, from 22% to 27%, while the proportion of localized cases did not change, and the proportion of regional cases decreased. These authors point out that these results indicate a true increase in risk, because if the increase had reflected earlier detection due to wider use of colonoscopy, earlier stage at diagnosis would be expected. [31]
Prognosis
The approximate 5-year survival rate for colorectal cancer patients in the United States (all stages included) is 64.6%. [23] Survival is inversely related to stage: approximate 5-year relative survival rates are as follows:
-
Localized disease: 90.2%
-
Regional disease: 71.8%
-
Distant disease: 14.3%
A study by Chua et al found that approximately one in every three patients who undergo resection for colorectal liver metastases become actual 5-year survivors. [32] Of those, approximately half survive 10 years and are cured of colorectal liver metastases. A multivariate analysis of 1001 patients who underwent potentially curative resection of liver metastases identified five factors as independent predictors of worse outcome [33] :
-
Size greater than 5 cm
-
Disease-free interval of less than a year
-
More than one tumor
-
Primary lymph-node positivity
-
Carcinoembryonic antigen (CEA) level greater than 200 ng/mL
Aggarwal et al found that circulating tumor cells measured at baseline after the initiation of new therapy in patients with metastatic colorectal cancer independently predicted survival; in patients with a baseline carcinoembryonic antigen (CEA) value of 25 ng/mL or higher, those with low baseline levels of circulating tumor cells (< 3) had longer survival. Both the number of circulating tumor cells and the CEA level measured at 6-12 weeks independently predicted survival. [34]
Research suggests a role for intra-tumoral immune response as a predictor of clinical outcome in patients with colorectal cancer, in addition to more traditional pathological and molecular markers. Katz et al reported that in patients with colorectal liver metastases, high numbers of T regulatory cells relative to CD4 or CD8 T cells predicted poor outcome [35]
A study by Yothers et al found that black patients with resected stage II and stage III colon cancer had worse overall and recurrence-free survival compared with white patients who underwent the same therapy. Five-year overall survival rate was 68.2% for blacks and 72.8% for whites; the three-year recurrence-free survival was 68.4% in blacks and 72.1% in whites. [36]
A study by Campbell et al found that prediagnosis body mass index (BMI) is an important predictor of survival among patients with nonmetastatic colorectal cancer, whereas postdiagnosis BMI is not. [37] A separate study from Campbell et al found that spending 6 or more hours per day sitting was associated with higher all-cause mortality compared with sitting less than 3 hours per day. The study concluded that increased recreational physical activity in patients with colorectal carcinoma reduces mortality. [38]
Morikawa et al reported that in patients with colorectal cancer that tested negative for cadherin-associated protein β 1 (CTNNB1 or β-catenin), high physical activity (≥18 metabolic equivalent task [MET] hours/week) after diagnosis was associated with significantly better cancer-specific survival. No association between physical activity and survival was seen in CTNNB1–positive cases. [39]
A review of eight trials by Rothwell et al found that allocation to aspirin reduced death caused by cancer. Benefit was apparent after 5 years of follow-up. The 20-year risk of cancer death was also lower in the aspirin group for all solid cancers. A latent period of 5 years was observed before risk of death was decreased for esophageal, pancreatic, brain, and lung cancers. A more delayed latent period was observed for stomach, colorectal, and prostate cancer. The overall effect on 20-year risk of cancer death was greatest for adenocarcinomas. [40]
A study by Burn et al found that 600 mg of aspirin per day for a mean of 25 months reduced cancer incidence after 55.7 months among known carriers of hereditary colorectal cancer. However, further studies are needed to determine the optimum dose and duration of treatment. [41]
Patients with preexisting mental disorders have an overall higher mortality rate than their counterparts. This higher mortality rate can be attributed to a lack of surgery, chemotherapy, and radiation therapy, especially in patients with psychotic disorders and dementia. Improved public health initiatives are needed to improve colon cancer detection and treatment in older adults with mental disorders. [42]
A study by Phipps et al found that smoking is also associated with increased mortality after colorectal cancer diagnosis, especially in patients whose cancer has high microsatellite instability. [43] A study by Dehal et al found that patients with colorectal cancer and type 2 diabetes mellitus have a higher risk of mortality than those without, most notably a higher risk due to cardiovascular disease. [44]
-
Standard colectomies for adenocarcinoma of the colon.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Guidelines
- Medication
- Medication Summary
- Antineoplastic Agent, Antimetabolite (pyrimidine analog)
- Antidote, Folic Acid Antagonist
- Antineoplastic Agent, Miscellaneous
- Antineoplastic Agent, Alkylating Agent
- Antineoplastic Agent, Monoclonal Antibody
- Antineoplastics, Tyrosine Kinase Inhibitors
- Antineoplastics, VEGF Inhibitor
- PD-1/PD-L1 Inhibitors
- Antineoplastics, Other
- Antineoplastics, BRAF Kinase Inhibitor
- Show All
- Questions & Answers
- Tables
- References