Background
Cyclospora cayetanensis (8-10 µm in diameter), a coccidian, obligate intracellular, protozoan parasite, produces an intestinal infection (called cyclosporiasis) in nonimmune persons that is ultimately self-limited (lasting up to 7-9 wk) and characterized by cyclical diarrhea (explosive at times; up to numerous times per day), accompanied by fatigue, malaise, anorexia, nausea, weight loss, and abdominal cramping and interspersed with periods of remission. [1, 2] It may be preceded by a flulike prodrome. Low-grade fever and malabsorption (as demonstrated by a D-xylose test) may occur. The diarrhea may continue for weeks to months if left untreated. Cyclospora infection affects both immunocompetent and immunocompromised individuals, the latter potentially more severely (ie, chronic, relapsing, protracted symptoms). The only consistently effective treatment is with trimethoprim-sulfamethoxazole (TMP-SMZ).
Cyclospora was first reported in Papua New Guinea in 1979 as an oocystlike body found in 3 patients with intestinal infections. It wasn't until the early 1990s that it became fully identified. [1] From 1986-1991, several reports described diarrhea associated with a large "Cryptosporidium" or cyanobacteriumlike bodies in both immunocompetent and immunosuppressed patients from North, Central, and South America; the Caribbean; Nepal; India; and Southeast Asia. Since the 1990s, increased globalizartion of food has enabled spread of Cyclospora to nonendemic areas such as the United States and many parts of Europe. [3] Shlim et al reported on the largest series of cases (55) from the CIWEC Clinic Travel Medicine Center in Katmandu, Nepal. [4]
In 1993, in Lima, Peru, Ortega et al characterized and clarified remaining taxonomic issues for C cayetanensis. [5] Also in 1993, a prospective study of 1042 stool specimens in patients with diarrhea at the Lahey Clinic in Massachusetts yielded 3 patients with Cyclospora infection. In the late spring and early summer of 1996, an outbreak affecting approximately 1450 individuals (70% laboratory confirmed) was described in Canada and the United States. [6] Since then, numerous reports have documented its endemicity in 27 countries around the world.
Table 1. Epidemiology of C cayetanensis * (Open Table in a new window)
Pattern of Spread |
Countries |
Comments |
Endemic |
Bangladesh, Brazil, Chile, China, Cuba, Dominican Republic, Egypt, Guatemala, Haiti, India, Indonesia, Jordan, Mexico, Morocco, Nepal, Nigeria, Pakistan, Peru, Puerto Rico, Romania, Saudi Arabia, Tanzania, Thailand, Turkey, Venezuela, Viet Nam, Zimbabwe |
Prevalence (1-15%†) varies significantly with the season and from year to year; children (≤ 9 y, most studies) account for 70-80% cases, which are typically asymptomatic (72-94%); asymptomatic disease is higher in older children (10-18 y) and adults (>18 y); infection rate in those with HIV is significantly higher than overall prevalence |
International travel-related |
Australia, Belgium, Czech Republic, Germany, Greece, Ireland, Italy, Japan, The Netherlands, Spain, Switzerland, United Kingdom, United States |
≤4% returning travelers with diarrhea |
Foodborne outbreaks |
Canada, United States, Germany, Mexico |
Canada/United States: raspberries, blackberries, mesclun, basil‡; Germany: lettuce imported from Southern France/Southern Italy; Mexico: watercress |
Waterborne outbreaks |
United States (Chicago), Nepal |
14 cases of cyclosporiasis; tap water in medical dormitory, suspected source was contaminated water storage tank; 12 of 14 developed cyclosporiasis |
* Community-based studies † Highest in spring and early summer ‡ Fresh produce. Raspberries, peas, or blackberries from Guatemala; mesclun (young salad greens, eg, spring mix, field greens, baby greens, gourmet salad mix) or lettuce from Peru or United States; basil from Mexico or United States. |
||
Transmission occurs primarily through ingesting contaminated food (eg, fruits, vegetables) and water. Fecal-oral transmission has also been suggested. No documented human-to-human transmission exists. [1]
Pathophysiology
Characteristic of coccidia (phylum Apicomplexa), sporozoites of Cyclospora within the sporocyst have a membrane-bound nucleus and micronemes.
Cyclospora undergo both sexual and asexual reproduction. They appear microscopically as nonrefractile, double-walled spheres 8-10 µm in diameter. On a modified acid-fast stain, the organism stains variably acid-fast because some organisms resist staining ("ghosts"). Cyclospora fluoresces blue under ultraviolet light.
Cyclospora is a small bowel pathogen. After ingestion, Cyclospora oocysts excyst in the GI tract and invade small bowel epithelia, where they undergo asexual division followed by sexual division, and produce mature oocysts that are shed in the stool.
Grossly, moderate to severe erythema of the distal duodenum is observed in patients with Cyclospora infection. Distal duodenal histopathological findings include acute and chronic inflammation, reactive hyperemia with vascular dilatation and villous capillary congestion, parasitophorous vacuoles that contain both asexual and sexual forms, crypt hyperplasia, epithelial disarray, and partial villous atrophy. Electron micrographs have demonstrated intracellular particles similar to sporozoites.
Abnormal findings on lactulose or mannitol studies or studies of both have demonstrated intestinal barrier disruption. Abnormal findings on D-xylose studies have demonstrated malabsorption. The nature of the immune response to Cyclospora is unclear, and only a few observations can be made. Patient sera have demonstrated Cyclospora -specific antibodies. Long-term expatriates tend to have fewer recurrences than short-term expatriates. In Haiti, patients with AIDS have recurrent disease. [7]
C cayetanensis infection occurs only in humans (ie, not in other animals). Of the 16 known Cyclospora species that infect animals (primates, other mammals, reptiles), none infect humans. Therefore, no animal reservoir for C cayetanensis is known or suspected. While this Cyclospora species only infects humans, animals, such as dogs, chickens, and various primates, can be paratenic hosts (ie, transient carriers), but the effect of this on human infection is not well studied.126 Cyclospora does not survive in biosolids (soil-like residue removed from sewage during the treatment process) secondary to heat of the process. Cyclospora has been demonstrated in source waters in several countries.6 It has also been isolated from wastewater in Tunisia and in Arizona.78
In endemic countries, soil contact is an important risk factor for children younger than 2 years. Oocysts can survive in water for 2 months at 39.2°F (4°C) and for 7 days at 98.6°F (37°C). Heating them at 140°F (60°C) for 60 minutes prevents sporulation. Freezing them at -0.4°F (-18°C) prevents sporulation. Desiccation for 15 minutes ruptures the oocyst wall. They are resistant to chlorine disinfection at standard water treatment levels. Pesticides at recommended levels (fungicides: Captan 50% WP, benomyl 50% WP, zineb 75% WP; insecticides: malathion 25% WP, diazinon 4E 47.5%) do not affect sporulation. Washing contaminated vegetables does not completely remove all of the sporocysts.
In endemic countries, the prevalence (1-15%) varies with the season (usually highest in spring and early summer) and from year to year in the same locale. Children (< 10-20 y, depending on the study) account for about 70% of infections, and 72-94% of these children are asymptomatic. Some adults in endemic countries have asymptomatic infections as well but do not excrete many oocysts. These observations suggest the possibility of a carrier state, but the certainty of this is far from demonstrated. Although more is becoming known about the biology of C cayetanensis, it remains unclear how the organism persists in the environment.
Life cycle of Cyclospora
Humans ingest sporulated oocysts (the infectious stage) of C cayetanensis, which only infects humans. The oocyst excysts in the small intestine, usually in the jejunum, and invades the intestinal epithelial cells. The next process is schizogony, which begins with the formation of a trophozoite that grows into a mature schizont that contains 8-12 merozoites, which are then released, presumably by cell rupture, to invade other epithelial cells and repeat the process. These merozoites are called type I meronts, which are asexual forms.
After several cycles of type I schizogony, type II meronts (sexual forms) develop, with each cell containing 4 merozoites. After invading epithelial cells, some of these form single macrogametes and others divide multiple times to form microgametes. When released, a microgamete fertilizes a macrogamete, which develops into a zygote. The zygote, in turn, develops into an oocyst with an environmentally resistant wall. The oocyst passes into the environment in the feces, as a nonsporulated noninfectious oocyst.
Consequently, human-to-human transmission does not occur. During infection, best evidence suggests that oocysts are continuously excreted. In the environment, the oocyst sporulates, becoming infectious for humans. During sporulation, the sporont divides into 2 sporocysts, each containing 2 sporozoites. Time course in the environment is days to weeks. In culture, 10-20% of sporonts have completed the process in 5 days. In other experimental studies, sporulation at ambient temperature occurs in 7-12 days. The preferred temperature is 78.8-86°F (26-30°C). Contamination of food or drinking water leads to human ingestion and infection. The infectious inoculum is small but has not been precisely quantitated.
Cyclosporiasis is seasonal in Guatemala (May through August), Haiti (January through March or April), Nepal (May through August), and Peru (December through May), often disappearing for months at a time.
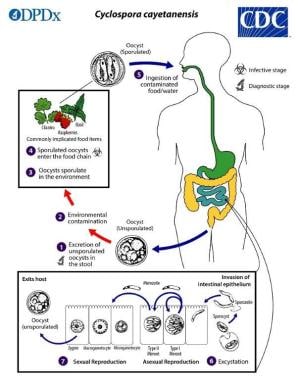 Life cycle of Cyclospora cayetanensis in pictorial form. Courtesy of CDC/DPDx [https://www.cdc.gov/parasites/cyclosporiasis/biology.html].
Life cycle of Cyclospora cayetanensis in pictorial form. Courtesy of CDC/DPDx [https://www.cdc.gov/parasites/cyclosporiasis/biology.html].
Epidemiology
Frequency
United States
C cayetanensis causes an estimated 16,264 cases of foodborne illness in the United States each year out of the estimated 76 million cases of foodborne illness overall (325,000 hospitalizations; 5,000 deaths). No deaths have been reported secondary to Cyclospora infection (CDC data). [8]
A decreasing incidence of cyclosporiasis has been reported in the United States (34 states reporting infections) from May to early August 2020 (1241 cases vs 2,408 cases during the same period in 2019). There has typically been an increase in cases over the past few years, so this decrease may be a reflection of the COVID-19 pandemic. [9]
The disease rate after a presumed exposure has been reported to vary from 32.5-100%, with a median of 91.7%, suggesting that a small inoculum of organisms is sufficient to infect.
It is a cause of travelers' diarrhea in a small percentage of travelers returning to developing countries.
It has been reported as a cause of foodborne diarrhea in outbreaks secondary to imported food (eg, raspberries, mesclun, basil) in the United States (see Table). In recent years, there have been outbreaks due to domestic contamination from different salad mixes, vegetable trays, and produce like romaine lettuce and green onions . [10, 11] [12]
International
C cayetanensis has been reported as endemic in at least 27 countries, mostly tropical (see Table). [13, 14, 15, 16, 17, 18, 19] Prevalence of cyclosporiasis can range from 3.3% in Mexico to as high as 41.6% in Peru; a recent large study examining prevalence rates in many countries [3, 20, 21] The average prevlance of Cyclospora in fuits and vegetables in many countries is around 3.5%. [22]
It has been reported as a cause of travelers' diarrhea after international travel by at least 11 countries such as Spain, Netherlands, and UK . [3]
It has been reported as a cause of foodborne diarrhea in outbreaks secondary to food imported to Canada (eg, raspberries, mesclun, basil) and Germany (lettuce) (see Table).
Europe is not endemic to Cyclospora, but it can be found there via importation of berries, green vegetales, and water; one study noted 1.4% of berries imported to Europe are contaminated with Cyclospora. One study examining Colombian strawberries noted a colonization rate of .83%. [3, 23]
It has been reported as a cause of an outbreak in Mexico secondary to watercress, cilantro, and premade salad mixes (within the country ). [19]
Mortality/Morbidity
Cyclospora infection is not considered a fatal disease. No reported deaths have been directly attributed to it in the United States. The greatest risk comes from dehydration in susceptible hosts.
Infants are at risk for critical dehydration due to protracted diarrhea. Worldwide diarrheal disease, in general, is responsible for more than 2 million deaths in children each year, mostly in developing countries. The percentage of these deaths that might be attributable to Cyclospora infection is not currently known.
A protracted course of several weeks to months with diarrhea, dehydration, and weight loss can produce significant morbidity. It is more severe in the immunologically naive, such as expatriates and travelers. In endemic countries, children often have asymptomatic infections (about 70%), and adults are infrequently infected.
If exposed to Cyclospora, patients with HIV who are not taking TMP-SMZ for prophylaxis have a significant risk for developing chronic and debilitating diarrhea.
Race
No racial predilection exists.
Sex
No sex predilection exists.
Age
In endemic countries, infections are much more common in children younger than 10-15 years (about 80% of infections). In this group, infections tend to be less frequent than in infants younger than 12-18 months (see Table). Severe clinical presentation may occur in elderly adults. [24]
-
Photomicrograph of a fresh stool sample prepared with a 10% formalin solution and stained with modified acid-fast stain. This image shows four Cyclospora cayetanensis oocysts with variable staining among the four oocysts. Courtesy of CDC/DPDx - Melanie Moser [https://phil.cdc.gov/Details.aspx?pid=7827].
-
Photomicrograph of a fresh stool sample prepared using a 10% formalin solution and stained with safranin. Three homogenously stained Cyclospora cayetanensis oocysts can be seen. Courtesy of CDC/DPDx - Melanie Moser [https://phil.cdc.gov/Details.aspx?pid=7828].
-
Life cycle of Cyclospora cayetanensis in pictorial form. Courtesy of CDC/DPDx [https://www.cdc.gov/parasites/cyclosporiasis/biology.html].







