Practice Essentials
Through their secretion of parathyroid hormone (PTH), the parathyroid glands are primarily responsible for maintaining extracellular calcium concentrations. Hyperparathyroidism is a disease characterized by excessive secretion of parathyroid hormone, an 84–amino acid polypeptide hormone. The secretion of parathyroid hormone is regulated directly by the plasma concentration of ionized calcium. See the image below.
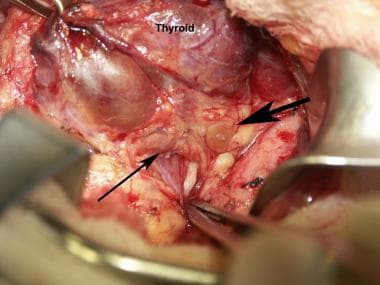 Normal parathyroid glands as seen during a thyroidectomy. The large arrow points to the superior parathyroid. The thinner arrow points to the inferior parathyroid. The forceps points toward the recurrent laryngeal nerve. The patient's head is toward the top right.
Normal parathyroid glands as seen during a thyroidectomy. The large arrow points to the superior parathyroid. The thinner arrow points to the inferior parathyroid. The forceps points toward the recurrent laryngeal nerve. The patient's head is toward the top right.
The main effects of parathyroid hormone are to increase the concentration of plasma calcium by increasing the release of calcium and phosphate from bone matrix, increasing calcium reabsorption by the kidney, and increasing renal production of 1,25-dihydroxyvitamin D-3 (calcitriol), which increases intestinal absorption of calcium. Thus, overproduction of parathyroid hormone results in elevated levels of plasma calcium. Parathyroid hormone also causes phosphaturia, thereby decreasing serum phosphate levels. Hyperparathyroidism is usually subdivided into primary, secondary, and tertiary hyperparathyroidism.
Signs and symptoms of hyperparathyroidism
Primary hyperparathyroidism
Skeletal manifestations include a selective cortical bone loss. Bone and joint pain, pseudogout, and chondrocalcinosis have also been reported.
Renal manifestations include the following:
-
Polyuria
-
Kidney stones
-
Hypercalciuria
-
Nephrocalcinosis (rarely)
Gastrointestinal manifestations include the following:
-
Vague abdominal pain
-
Anorexia
-
Nausea
-
Vomiting
-
Constipation
-
Peptic ulcer disease
-
Acute pancreatitis
Neuromuscular and psychological manifestations include the following:
-
Fatigue
-
Muscle weakness
-
Depression
-
Inability to concentrate
-
Memory problems or subtle deficits
Cardiovascular manifestations include the following [1] :
-
Hypertension
-
Bradycardia
-
Shortened QT interval
-
Left ventricular hypertrophy
Workup in hyperparathyroidism
Primary hyperparathyroidism
Testing of the intact parathyroid hormone level is the core of the diagnosis. An elevated intact parathyroid hormone level with an elevated ionized serum calcium level is diagnostic of primary hyperparathyroidism. A 24-hour urine calcium measurement is necessary to rule out familial benign (hypocalciuric) hypercalcemia (FHH).
Ultrasonography of the neck is a safe and widely used technique for localization of abnormal parathyroid glands.
Secondary hyperparathyroidism
The serum level of parathyroid hormone, calcium, phosphorus, and 25-hydroxyvitamin D should be measured. Patients with secondary hyperparathyroidism usually have low-normal calcium and elevated parathyroid hormone. The serum phosphorus level may vary based on the etiology, trending towards higher values with reduced kidney function and lower values with vitamin D deficiency.
Ultrasonography may reveal thyroid pathology that was previously not recognized and that should be dealt with at the time of neck exploration.
Management of hyperparathyroidism
Primary hyperparathyroidism
Surgical excision of abnormal parathyroid glands offers the only permanent, curative treatment for primary hyperparathyroidism.
Secondary hyperparathyroidism
Unlike primary hyperparathyroidism, medical management is the mainstay of treatment for secondary hyperparathyroidism.
Nonsurgical options for the management of secondary hyperparathyroidism in chronic kidney disease (CKD) include the following:
-
Dietary phosphorus restriction
-
Phosphate binders
-
Vitamin D and its analogs
-
Calcimimetics
Tertiary hyperparathyroidism
Total parathyroidectomy with autotransplantation or subtotal parathyroidectomy is indicated.
Anatomy and Embryology
Usually, 4 parathyroid glands are situated posterior to the thyroid gland. A small number of patients have 3, 5, or, occasionally, more glands. The glands are identified based on their location as right or left and superior or inferior.
The inferior glands are derived from the third pharyngeal pouch. This structure is also the embryologic origin of the thymus. Therefore, the inferior glands originate more cephalad than the superior glands, but they migrate along with the thymus to finally become situated more inferiorly than the superior glands. Because of their embryologic association with the thymus, the inferior glands are often found adjacent to or within the thymus. They are usually located near the inferior pole of the thyroid.
The superior glands are more consistent in location, usually found just superior to the intersection of the inferior thyroid artery and the recurrent laryngeal nerve. The superior glands are derived embryologically from the fourth pharyngeal pouch. This structure also gives rise to the C cells of the thyroid gland. Because of their embryologic origin, the superior glands are occasionally found within the substance of the thyroid gland. Ectopic locations of parathyroid glands are discussed in more detail in Surgical care in Primary Hyperparathyroidism.
Primary Hyperparathyroidism
Definition of problem
Primary hyperparathyroidism is the unregulated overproduction of parathyroid hormone (PTH) resulting in abnormal calcium homeostasis.
Frequency
Primary hyperparathyroidism is more common in women, the incidence being 66 per 100,000 person-years in females, and 25 per 100,000 person-years in males. In a large study of 3.5 million enrollees in Kaiser Permanente of southern California, the incidence fluctuated over time but was not seen to decrease substantially. On the contrary, the prevalence of primary hyperparathyroidism saw a substantial increase in this population. [2] The mean age at diagnosis has remained between 52 and 56 years.
Etiology
In approximately 85% of cases, primary hyperparathyroidism is caused by a single adenoma. In 15% of cases, multiple glands are involved (ie, either multiple adenomas or hyperplasia). [3] Rarely, primary hyperparathyroidism is caused by parathyroid carcinoma. The etiology of adenomas or hyperplasia remains unknown in most cases. Familial cases can occur as either part of the multiple endocrine neoplasia syndromes (MEN 1 or MEN 2a), hyperparathyroid-jaw tumor (HPT-JT) syndrome, or familial isolated hyperparathyroidism (FIHPT). Familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism also belong to this category. The molecular genetic basis of MEN 1 is an inactivating mutation of the MEN1 gene, located on chromosome band 11q13. MEN 2a is caused by a germline mutation of the Ret proto-oncogene on chromosome 10. [4] Germline mutation of HRPT2 localized on chromosome arm 1q is responsible for HPT-JT, while FIHPT is genetically heterogeneous.
Pathophysiology
In primary hyperparathyroidism due to adenomas, the normal feedback on parathyroid hormone production by extracellular calcium seems to be lost, resulting in a change in the set point. However, this is not the case in primary hyperparathyroidism from parathyroid hyperplasia. An increase in the cell numbers is probably the cause.
The chronic excessive resorption of calcium from bone caused by excessive parathyroid hormone can result in osteopenia. In severe cases, this may result in osteitis fibrosa cystica, which is characterized by subperiosteal resorption of the distal phalanges, tapering of the distal clavicles, salt-and-pepper appearance of the skull, and brown tumors of the long bones. This is not commonly seen now. In addition, the chronically increased excretion of calcium in the urine can predispose to the formation of renal stones.
The other symptoms of hyperparathyroidism are due to the hypercalcemia itself and are not specific to hyperparathyroidism. These can include muscle weakness, fatigue, volume depletion, nausea and vomiting, and in severe cases, coma and death. Neuropsychiatric manifestations are particularly common and may include depression, confusion, or subtle deficits that are often characterized poorly and may not be noted by the patient (or may be attributed to aging). Increased calcium can increase gastric acid secretion, and persons with hyperparathyroidism may have a higher prevalence of peptic ulcer disease. Rare cases of pancreatitis have also been attributed to hypercalcemia.
A prospective cohort study by Ejlsmark-Svensson et al reported that in patients with primary hyperparathyroidism, quality-of-life questionnaire scores were significantly lower in association with moderate-severe hypercalcemia than in relation to mild hypercalcemia. However, quality of life did not seem to be related to the presence of organ-related manifestations of primary hyperparathyroidism, such as osteoporosis, renal calcifications, and renal function impairment. [5] This suggests that hypercalcemia is the primary driver of an impaired quality of life.
Clinical presentation
History
The clinical syndrome of primary hyperparathyroidism can be easily remembered as "bones, stones, abdominal groans, and psychic moans." With the introduction of routine measurement of blood calcium in the early 1970s, the most common clinical presentation of primary hyperparathyroidism changed from severe bone disease or kidney stones to asymptomatic hypercalcemia. [6]
Skeletal manifestations of primary hyperparathyroidism include primarily a selective cortical bone loss. Bone and joint pain, pseudogout, and chondrocalcinosis have also been reported. In the early clinical descriptions of primary hyperparathyroidism, some patients developed a peculiar type of bone disease known as osteitis fibrosa cystica, which was characterized by increased generalized osteoclastic bone resorption. Radiographic plain film changes associated with osteitis fibrosa cystica include subperiosteal resorption in the phalanges and a finding known as salt and pepper skull. This presentation is rarely seen today except in medically underserved populations.
Renal manifestations include polyuria, kidney stones, hypercalciuria, and, rarely, nephrocalcinosis.
Gastrointestinal manifestations include vague abdominal pain, anorexia, nausea, vomiting, constipation, peptic ulcer disease, and acute pancreatitis.
Neuromuscular and psychological manifestations include fatigue, muscle weakness, depression, inability to concentrate, and memory problems or subtle deficits that are often characterized poorly and may not be noted by the patient, a common description being “brain fog." These symptoms are often attributed to aging, and some patients are diagnosed with chronic fatigue syndrome or fibromyalgia.
Cardiovascular manifestations include hypertension, bradycardia, shortened QT interval, and left ventricular hypertrophy. [1]
Physical
Physical examination findings are usually noncontributory. Examination may reveal muscle weakness and depression. A palpable neck mass is not usually expected with hyperparathyroidism, although in rare cases, it may indicate parathyroid cancer. A previously undiagnosed thyroid nodule is much more commonly the source of a palpable nodule.
Diagnostic considerations
The causes of hypercalcemia that result in a concomitantly elevated parathyroid hormone level are few. These include familial benign (hypocalciuric) hypercalcemia (FHH) (see Related disorders), lithium-induced hypercalcemia, and tertiary hyperparathyroidism. A minority of patients (ie, 10-15%) with hyperparathyroidism have parathyroid hormone levels that are within the reference range but are inappropriately high in the presence of elevated serum calcium concentrations. A subset of patients has normal calcium levels with elevated parathyroid hormone, so-called normocalcemic hyperparathyroidism. However, when considering this diagnosis, all potential causes of secondary hyperparathyroidism (eg, low calcium intake, gastrointestinal disorders, renal insufficiency, vitamin D deficiency, hypercalciuria of renal origin) should be excluded. Patients with normal calcium levels and elevated parathyroid hormone levels in the absence of an identifiable secondary cause should be monitored for progression to hypercalcemia.
Secondary and tertiary hyperparathyroidism are typically diagnosed based on their clinical context. Cancer-induced hypercalcemia is associated with a low parathyroid hormone level but possibly a high parathyroid hormone–related peptide level.
Workup
Laboratory studies
Total serum calcium and albumin levels or ionized calcium levels should be measured. Hypercalcemia should be documented on more than one occasion before a diagnostic workup is undertaken.
Testing of the intact parathyroid hormone level is the core of the diagnosis. An elevated intact parathyroid hormone level with an elevated ionized serum calcium level is diagnostic of primary hyperparathyroidism. A 24-hour urine calcium measurement is necessary to rule out FHH.
Older assays measured fragments of the parathyroid hormone molecule, such as the C-terminal or mid region of parathyroid hormone. These first-generation assays are considered obsolete for the clinical practice. Second-generation parathyroid hormone assays, globally called "intact" parathyroid hormone assays, and third-generation parathyroid hormone assays called "whole" or "biointact" parathyroid hormone assays use two different antibodies against two different segments of parathyroid hormone. Second- and third-generation parathyroid hormone assays are producing far more clinically satisfying data than the first-generation assays but have some limitations that continue to be assessed in several studies.
Other laboratory findings in primary hyperparathyroidism include mild hyperchloremic acidosis, hypophosphatemia, and mild to moderate increase in urinary calcium excretion rate.
Vitamin D levels should be measured in the evaluation of primary hyperparathyroidism. Vitamin D deficiency (a 25-hydroxyvitamin D level of less than 20 ng per milliliter) can cause secondary hyperparathyroidism, and repletion of vitamin D deficiency can help to reduce parathyroid hormone levels. [7] In most studies, increasing serum 25-hydroxyvitamin D stores to at least 37.5 ng per milliliter is sufficient for parathyroid hormone suppression and prevention of secondary hyperparathyroidism in persons with normal renal function (although some studies have suggested increasing stores to 50 ng per milliliter). [8]
Imaging studies
Imaging studies are not used to make the diagnosis of primary hyperparathyroidism (which is based on laboratory data) or to make a decision about whether to pursue surgical therapy (which is based on clinical criteria). Imaging studies are used to guide the surgeon once surgical therapy has been decided. If a limited parathyroid exploration is to be attempted, a localizing study is necessary. Other uses of imaging studies in the initial evaluation of a patient with primary hyperparathyroidism are controversial (see Choice of surgical treatment, below).
For many patients, the recommendation remains for complete parathyroid exploration with resection of all involved glands. Many surgeons agree that imaging studies are not required when this surgical treatment is chosen. However, in patients who have recurrent or persistent hyperparathyroidism after a previous surgical exploration, an imaging test to localize involved glands is definitely indicated.
Ultrasonography of the neck is a safe and widely used technique for localization of abnormal parathyroid glands. It is capable of a high degree of accuracy, but it is operator dependent and its reported accuracy has varied widely in the literature. One advantage of neck ultrasonography is that it can be performed rapidly by the clinician at the time of the initial evaluation. Studies of clinician-performed ultrasonography show accuracy rates that compare favorably with the accuracy of traditional radiology departments, in the vicinity of 75-80%. [9, 10, 11, 12] Ultrasonography, like nuclear medicine scanning, has not been reliable in detecting multigland disease.
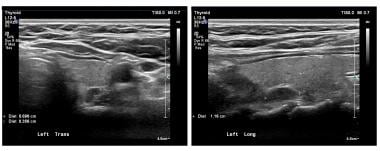 The panels show a transverse and longitudinal (sagittal) view of a left superior parathyroid adenoma on ultrasonography. The caliper marks designate the adenoma. Adenomas are typically homogeneous and hypoechoic.
The panels show a transverse and longitudinal (sagittal) view of a left superior parathyroid adenoma on ultrasonography. The caliper marks designate the adenoma. Adenomas are typically homogeneous and hypoechoic.
Nuclear medicine scanning with radiolabeled sestamibi is also a widely used technique. Sestamibi is commonly used in cardiac imaging and was found serendipitously to accumulate in parathyroid adenomas. This radionuclide is concentrated in thyroid and parathyroid tissue but usually washes out of normal thyroid tissue in under an hour. It persists in abnormal parathyroid tissue. See the image below.
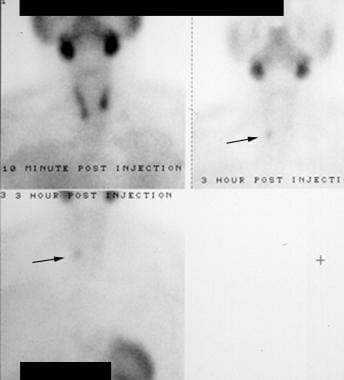 Hyperparathyroidism. Technetium-99m (99mTc) sestamibi radionuclide scan. The early image (top left) shows uptake in the salivary glands and thyroid. The later images (right and bottom) show washout from the thyroid but persistence in the region of the right inferior thyroid lobe (arrows). This proved to be a right parathyroid adenoma.
Hyperparathyroidism. Technetium-99m (99mTc) sestamibi radionuclide scan. The early image (top left) shows uptake in the salivary glands and thyroid. The later images (right and bottom) show washout from the thyroid but persistence in the region of the right inferior thyroid lobe (arrows). This proved to be a right parathyroid adenoma.
On delayed images, an abnormal parathyroid is seen as a persistent focus of activity. The scan's sensitivity for detecting solitary adenomas has varied widely in the literature but generally is reported as 60-90%. The main weakness of this test is in diagnosing multiglandular disease. In this case, sensitivity drops to approximately 50%. [3] Most modern sestamibi scans are performed with single-photon computed tomography (SPECT). This technique (see the image below) combines the detection of the radioactivity with the detailed imaging of CT scanning, allowing better sensitivity and more precise anatomic localization than standard planar imaging (as shown above).
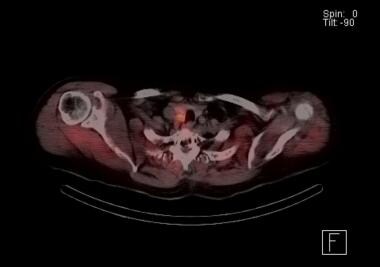 Sestamibi parathyroid scan with SPECT scan. The orange indicates radionuclide accumulation. The findings indicate the presence of a right-sided parathyroid adenoma just behind the thyroid lobe. At exploration, this patient was found to have adjacent double adenomas on the right.
Sestamibi parathyroid scan with SPECT scan. The orange indicates radionuclide accumulation. The findings indicate the presence of a right-sided parathyroid adenoma just behind the thyroid lobe. At exploration, this patient was found to have adjacent double adenomas on the right.
One major advantage of the sestamibi parathyroid scan is the ability to detect ectopic parathyroid glands, particularly in the mediastinum.
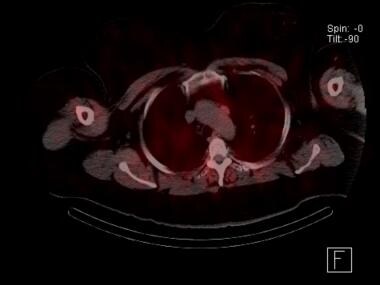 Sestamibi parathyroid scan with SPECT scan showing an ectopic mediastinal parathyroid adenoma adjacent to the aortic arch. This patient had undergone a failed neck exploration. A transthoracic, robotic excision was curative.
Sestamibi parathyroid scan with SPECT scan showing an ectopic mediastinal parathyroid adenoma adjacent to the aortic arch. This patient had undergone a failed neck exploration. A transthoracic, robotic excision was curative.
The use of four-dimensional (4D) CT scanning for parathyroid localization is increasing. [13] The study can be done either without contrast or with dynamic contrast imaging. Parathyroid adenomas enhance brightly with contrast due to their high vascularity, and then the contrast quickly washes out. Four-dimensional CT scan studies have shown sensitivity rates as high as 88%. [14, 15] The largest retrospective study available at the time of this writing reported an overall sensitivity of 79%. [16] Like other imaging studies, 4D-CT scanning is less sensitive in detecting multiglandular disease (43-67% [17, 18] ) than single-gland disease (92-94% [19, 20] ). Some studies have argued that a two-phase CT scan is as effective as the 4D modality in the localization of the parathyroids, including in cases of small adenomas, reoperation, and multiglandular disease, with less radiation exposure for the patient. [21] ) However, while the two-phase technique does lower radiation exposure, this is probably at the cost of optimal accuracy. [22, 20]
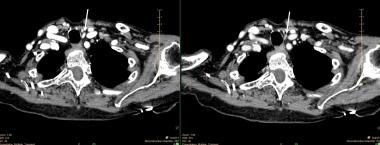 A small, left inferior parathyroid adenoma as demonstrated on a 4D-CT scan. The left panel is a single image from the early contrast phase showing intense enhancement. The right panel shows rapid washout of contrast. The white arrows point to the adenoma.
A small, left inferior parathyroid adenoma as demonstrated on a 4D-CT scan. The left panel is a single image from the early contrast phase showing intense enhancement. The right panel shows rapid washout of contrast. The white arrows point to the adenoma.
Magnetic resonance imaging (MRI) has not commonly been used for parathyroid localization in most centers, and studies regarding this modality have generally been small and have all utilized contrast. Newer techniques, conceptually similar to the 4D-CT scanning, are being developed that may increase the sensitivity of MRI and expand its usefulness. [23, 24, 25]
Dual-energy radiographic absorptiometry is a useful tool to demonstrate the skeletal involvement in primary hyperparathyroidism. Note that hyperparathyroidism preferentially affects the cortical bone at the radius (distal third). In cases of severe primary hyperparathyroidism, skeletal radiographs show pathognomonic changes such as salt-and-pepper degranulation in the skull and subperiosteal bone resorption in the phalanges. However, plain radiographs are generally not useful for routine employment in the diagnosis and treatment of hyperparathyroidism.
A study by Thimmappa et al suggested that imaging studies can be used in place of intraoperative parathyroid assays (discussed below) to predict cure in surgery for primary hyperparathyroidism. The investigators described the following protocol [26] :
-
Two preoperative localization studies, including one with surgeon-performed ultrasonography, are performed
-
Preoperative vitamin D levels are assessed, with supplementation provided as indicated
The report contended that in select patients with strong corroboration between the two localization studies and intraoperative findings that are consistent with these studies, intraoperative parathyroid assays may not be required, with the study finding that cure rates in patients in whom this protocol was employed equaled those attained using parathyroid assays. [26]
Procedures
Bilateral internal jugular vein sampling is used to help localize ectopic parathyroid adenomas, usually in cases of failed surgical exploration when standard imaging techniques have not been helpful. This technique should generally be reserved for centers with specialists and for highly selected patients.
Treatment
Surgical excision of abnormal parathyroid glands (see below for details of surgical treatment) offers the only permanent, curative treatment for primary hyperparathyroidism. There is universal agreement that surgical treatment should be offered to all patients with symptomatic disease. The authors would note, however, that symptoms are often dismissed by both physicians and patients; fatigue is an extremely common symptom that is often ignored, especially in the elderly, who commonly attribute it to aging or other causes. Some controversy exists regarding the optimal management of asymptomatic patients.
Guidelines for the management of asymptomatic primary hyperparathyroidism were updated in 2013 by the Fourth International Workshop on Asymptomatic Primary Hyperparathyroidism. Indications for surgery include the following [27] :
-
Serum calcium >1 mg/dL above the upper limit of the reference range
-
Bone mineral density T-score at or below -2.5 (in perimenopausal or postmenopausal women and in men aged 50 years or older) at the lumbar spine, total hip, femoral neck, or distal 1/3 radius
-
Vertebral fracture as evidenced via radiography or vertebral fracture assessment (VFA)
-
Creatinine clearance of < 60 cc/min
-
Twenty-four–hour urinary calcium excretion >400 mg/day and increased stone risk as assessed through biochemical stone risk analysis.
-
Presence of nephrolithiasis or nephrocalcinosis as determined using radiography, ultrasonography, or CT scanning
-
Age younger than 50 years
Some clinicians advocate surgical therapy in all patients with primary hyperparathyroidism, modified only for those patients who are not able to tolerate surgery. They argue that the operation is generally well tolerated, that such treatment prevents complications (eg, osteoporosis), and that it may reverse symptoms that patients often do not realize they have (eg, fatigue, mild depression). In addition, the monitoring of asymptomatic patients is expensive and cumbersome. This more liberal approach has been articulated by an expert group convened by the American Association of Clinical Endocrinologists and the American Association of Endocrine Surgeons. They concluded that "...operative management should be considered and recommended for all asymptomatic patients with PHPT [primary hyperparathyroidism] who have a reasonable life expectancy and suitable operative and anesthesia risk factors." [28] This proactive approach, as with all parathyroidectomies, should depend on the availability of an experienced, well-trained surgeon.
A survey study by Sharata et al of primary care providers in the United States found that only a minority of respondents showed firm familiarity with management strategies for primary hyperparathyroidism. The investigators found that 31% of the 109 clinicians who responded to the survey were familiar with the whole range of criteria regarding surgical intervention in asymptomatic patients and that 34% were able to accurately identify correct surveillance testing for patients being observed. Among patients under observation, only 16% underwent proper surveillance studies. [29]
Management of severe hypercalcemia in the acute setting
Reduction of elevated serum calcium can be accomplished by the use of intravascular volume expansion with sodium chloride and loop diuretics such as furosemide once the intravascular volume is restored. Drugs such as calcitonin and IV bisphosphonate have been used as a temporary measure prior to surgical treatment.
Nonsurgical care and long-term monitoring
Asymptomatic patients who do not undergo surgery require long-term monitoring. Recommendations include assessing for overt signs and symptoms of hyperparathyroidism annually, annual serum calcium and creatinine testing, and bone mineral density (spine, hip, and forearm) evaluation every 1-2 years. [27]
Patients with primary hyperparathyroidism should maintain a moderate daily elemental calcium intake of 800-1000 mg and a vitamin D intake appropriate for their age and sex. Maintaining good hydration, participation in regular exercise activity, and avoidance of immobilization and certain medications (such as thiazides, diuretics, and lithium) are desirable.
Pharmacotherapy
Estrogen therapy in postmenopausal women has been shown to cause a small reduction in serum calcium (0.5-1 mg/dL) without a change in parathyroid hormone. Estrogen also has beneficial effects of lumbar spine and femoral neck bone mineral density (BMD). However, due to risks associated with estrogen replacement, it should not be used for the sole purpose of treating primary hyperparathyroidism.
Selective estrogen receptor modulators such as raloxifene have been shown to cause a decrease in serum calcium of the same magnitude observed with estrogen.
Bisphosphonates, in particular alendronate, has been shown to improve the BMD at the spine and hip BMD in patients with primary hyperparathyroidism. [30, 31] No significant change in parathyroid hormone, calcium, or 24-hour urinary calcium has been reported. Treatment with a bisphosphonate such as alendronate can be considered in patients with primary hyperparathyroidism and low BMD who cannot, or will not, undergo surgery.
Calcimimetic drugs activate the calcium-sensing receptor and inhibit parathyroid cell function. [32, 33] Treatment with cinacalcet resulted in reduction without normalization of parathyroid hormone levels, reduction and even normalization of serum calcium, but no increase in BMD was observed.
Other treatments
Percutaneous alcohol injection, ablation with ultrasound energy, and other percutaneous ablation techniques of the parathyroid gland have been suggested as alternative treatments in patients with primary hyperparathyroidism who cannot or will not undergo surgery. Although studies of these techniques continue, their routine use cannot yet be supported.
Surgical care
Surgical treatment should be offered to most patients with primary hyperparathyroidism. The historical criterion-standard operative approach is complete neck exploration with identification of all parathyroid glands and removal of all abnormal glands. Approximately 85% of cases of primary hyperparathyroidism are caused by a single adenoma. Therefore, most patients who undergo full neck exploration to evaluate all parathyroids endure some unnecessary dissection. Rather than explore all parathyroid glands, a newer technique, directed parathyroidectomy, has evolved. This technique relies on preoperative imaging studies to localize the abnormal gland. The surgeon then removes only that gland, without visualizing the other glands.
With modern imaging techniques, an abnormal parathyroid may be detected preoperatively in 70-80% of cases. However, no current imaging study is reliable for detecting multiple abnormal glands. [3] Therefore, an additional method is required to confirm that no other abnormal glands are present after excision of the imaged lesion. For this purpose, most centers use the intraoperative parathyroid hormone assay. [3, 34, 35] Because the plasma half-life of parathyroid hormone is only approximately 4 minutes, the level falls quickly after resection of the source. If the level fails to fall after resection of the identified abnormal gland, the procedure is extended to allow for further exploration. However, the intraoperative parathyroid hormone assay is usually available only in centers that perform a high volume of parathyroidectomies.
A few authors have advocated radio-guided parathyroidectomy, detecting the labeled sestamibi in the abnormal gland using a handheld probe. Most centers have abandoned this technique because if the gland labels well with sestamibi, allowing for adequate preoperative imaging, use of the handheld probe intraoperatively is unnecessary in most cases.
Greene et al examined trends in surgeons' use of bilateral versus limited exploration for parathyroidectomy between 1998 and 2008. [36] Surveying 256 surgeons (members of the American Association of Endocrine Surgeons and the American College of Surgeons), who together accounted for 46% of parathyroid operations in the United States, the investigators found that in 2008, 10% of surgeons employed bilateral neck exploration, 68% used limited exploration, and 22% used both of these exploration techniques in their practice. In 1998, the statistics for surgeons using bilateral, limited, or both types of exploration were 74%, 11%, and 15%, respectively. The study indicated that the physicians who are most likely to use limited exploration are endocrine surgeons, surgeons with a high-volume practice, and surgeons whose mentors used limited exploration.
The authors also found that in 2008, half of the general surgeons surveyed never monitored parathyroid hormone intraoperatively (whether using bilateral or limited exploration), while the same held true for less than 10% of the endocrine surgeons. In addition, there was great variation "among subsets of surgeons in operative volumes, indications for bilateral neck exploration, [follow-up] care, expertise with [ultrasonography] and sestamibi, and perceptions of cure and complication rates." Greene and his coauthors concluded that because of the many differences that exist in the surgical management of hyperparathyroidism, best-practice guidelines may need to be defined.
For familial diseases, such as MEN 1, total parathyroidectomy is performed along with cervical thymectomy and autotransplantation to the forearm. Cryopreservation of some parathyroid tissue is also recommended. [37]
Parathyroidectomy is usually well tolerated. The main risks are injury to the recurrent laryngeal nerves and hypoparathyroidism due to resection or devascularization of all parathyroid glands. Although local anesthesia has been used successfully for this procedure, especially in the directed approaches during which a single adenoma is localized preoperatively, general anesthesia is used most commonly. In patients in whom hypercalcemia (and, therefore, dehydration) has been severe, special attention must be directed to perioperatively restoring the fluid balance. Neck mobility must be assessed to ensure proper positioning in the operating room.
Technique for full neck exploration with identification of all parathyroid glands
The most critical aspect to ensure success in this operation is identification of all 4 parathyroid glands and removal of all abnormal glands. In the case of 4-gland hyperplasia, a 3.5-gland (subtotal) parathyroidectomy is performed. Approximately 50-70 mg of the most normal-appearing tissue is left behind. A nonabsorbable suture is left as a tag to identify the gland should reoperation be necessary.
The patient is placed in the lawn-chair position with the neck extended over a transversely placed shoulder roll. This position allows full exposure of anterior neck structures and improves venous drainage.
A low transverse incision placed within a skin crease provides the best cosmetic result. The length of the incision must be adequate to allow thorough exploration of all potential locations of the parathyroid glands; however, given the elasticity of the neck skin flaps, a 2- to 5-cm incision usually allows safe identification of important structures.
After hemostasis of the skin incision is obtained, subplatysmal flaps are developed superiorly to the notch of the thyroid cartilage and inferiorly to the flat portion of the manubrium. The sternohyoid and sternothyroid (strap) muscles are separated in the midline to expose the thyroid gland. If preoperative localization studies suggest a parathyroid adenoma, that side is approached first.
Frequently, a middle thyroid vein may require ligation to ensure adequate mobilization of the thyroid lobe. The thyroid lobe is elevated off the common carotid artery and retracted medially. The inferior thyroid artery is identified after blunt and sharp dissection of the areolar tissue anteriorly and medially to the common carotid artery and posteromedially to the thyroid lobe. The recurrent laryngeal nerve is identified next, inferior and lateral to the lower lobe of the thyroid gland.
The intersection of the inferior thyroid artery and the recurrent laryngeal nerve is an important landmark because most parathyroid glands, superior and inferior, are located within 2 cm of this area. The superior parathyroid glands are located dorsal to the upper two thirds of the thyroid lobe and posterior to the recurrent laryngeal nerve. The inferior glands, which are less consistent in location, can usually be found inferior to the inferior thyroid artery and ventral to the recurrent laryngeal nerve. They are usually within 1 cm of the inferior lobe of the thyroid gland.
Occasionally, not all parathyroid glands can be identified. In such instances, the usual locations are reexamined first because most parathyroid glands are located in typical areas. If parathyroid glands are not identified in those locations, then a systematic search is performed, taking into consideration the path of descent of superior and inferior parathyroid glands.
Inferior glands may be located in the thyrothymic ligament. They may be difficult to identify, especially after division of the inferior thyroid vein, a maneuver that allows the gland to retract into the superior mediastinum. Another location for ectopic inferior parathyroid glands is the thymus. If an inferior gland cannot be located, a cervical thymectomy can be performed, elevating as much thymic tissue superiorly from the mediastinum as can be done safely.
Superior parathyroid glands are usually dorsal to the upper two thirds of the thyroid gland. Occasionally, these glands are adjacent to the superior thyroid vessels. Other locations include the carotid sheath or posterior to the esophagus or pharynx (retroesophageal). Finally, both superior and inferior parathyroid glands may be located aberrantly within the capsule of the thyroid gland. Some surgeons perform a thyroid lobectomy on the side of the missing abnormal gland after an exhaustive search is made in the aforementioned locations. Median sternotomy should generally not be performed during the initial neck exploration for hyperparathyroidism.
Abnormally enlarged glands are excised after confirmation of the normal size of other glands. During excision, avoiding capsular rupture of the abnormal gland is important because this may be associated with implantation of parathyroid cells in the operative site and subsequent parathyromatosis. Parathyroids may be identified by highly experienced surgeons based on appearance and location. If necessary, identification of the parathyroid glands should be confirmed histologically by frozen section examination. In cases of total parathyroidectomy with autotransplantation, parathyroid tissue should be cryopreserved for future autotransplantation in case the initial transplant does not function adequately.
Technique for directed parathyroidectomy
In many respects, the operative technique is similar to that described above for a complete parathyroid exploration. Differences are noted below.
Adequate imaging of the abnormal gland prior to surgery is essential. In addition, arrangements for intraoperative measurement of parathyroid hormone should be confirmed. [3] A line for sampling of peripheral venous blood should be established. Often, the distal saphenous vein provides the most convenient access.
Some surgeons modify the location of the incision based on the preoperative location of the adenoma. This author prefers a small incision (ie, ~2 cm) in the standard location for a collar incision. This incision can be readily extended should extensive exploration prove necessary.
A baseline parathyroid hormone level is drawn immediately prior to skin incision. Following identification and dissection of the adenoma, a preexcision level is drawn. Manipulation of the gland occasionally causes significant increases, sometimes of more than 10-fold, in the parathyroid hormone level. Following excision of the gland, parathyroid hormone levels are drawn at 5 minutes and 10 minutes postexcision. Criteria for adequate excision are either a 50% drop in parathyroid hormone from the baseline level to the 10-minute postexcision level or a 50% drop in parathyroid hormone from the preexcision level at 10 minutes and a postexcision level below the baseline level. [38]
The incision may be closed while the last parathyroid hormone levels are being processed, but the patient should remain under anesthesia, and the sterile field maintained until the parathyroid hormone assay results are known.
If a directed parathyroidectomy is performed successfully, most of these patients may be safely discharged the day of surgery.
Complications and postoperative care
For a full parathyroid exploration, postoperative hypoparathyroidism and hypocalcemia are concerns, but they are extremely uncommon after a directed parathyroidectomy and limited neck exploration. Hypocalcemia is more common after bilateral parathyroid exploration, especially when subtotal parathyroidectomy is performed. The nadir of serum calcium usually occurs 24-72 hours postoperatively. Many patients become hypocalcemic, but few become symptomatic. On the other hand, even when a limited exploration has been done, mild symptoms of hypocalcemia can occur in the first few days after parathyroidectomy in the absence of verifiable hypocalcemia. Because of this, some practitioners routinely administer oral calcium supplements postoperatively.
Hypocalcemia after parathyroid surgery may be due to hungry bone syndrome where calcium and phosphorus are rapidly deposited in the bone. This is characterized by hypoparathyroidism and transient, but occasionally severe, hypocalcemia until the normal glands regain sensitivity.
If hypoparathyroidism persists, oral supplementation with calcium and vitamin D is required. Calcium citrate or calcium carbonate may be started at 400-600 mg of elemental calcium four times per day. Some patients require substantially more. Calcitriol is started at 0.5 mcg twice daily and increased as required. Patients in whom total parathyroidectomy and autotransplantation is performed can be expected to require temporary calcium supplementation.
If a recurrent laryngeal nerve is transected during parathyroidectomy, immediate repair is indicated. If the recurrent nerve is not known to be injured intraoperatively but dysfunction is suggested because the patient has developed new hoarseness, expectant management is chosen initially since most patients recover nerve function over a few weeks to months. Laryngoscopy is indicated to document both dysfunction and recovery of function.
A potential life-threatening emergency in the postoperative period is the development of an expanding hematoma in the pretracheal space. This complication must be recognized and treated immediately by opening the wound and evacuating the hematoma. If untreated, laryngeal edema may progress rapidly, causing airway obstruction. Moreover, the edema may prevent endotracheal intubation, and opening of the wound should precede any intubation attempt.
Most small hematomas do not require treatment. A subplatysmal fluid collection may occasionally form, and these are usually treated adequately with a single aspiration. In a few cases, aspiration may need to be repeated. Rarely, a drain may need to be placed for recurrent fluid collections.
Treatment outcomes
Cure rates after surgery for primary hyperparathyroidism are very high in expert hands, approximately 97-98%. [39] A cure is generally defined as normalized serum calcium. Parathyroid hormone levels, however, may be elevated postoperatively in as many as 20-40% of patients. If the serum calcium remains within the reference range, this elevated state does not usually suggest persistent disease but may indicate a higher risk of recurrence. [40, 41, 42, 43, 44, 45, 46, 47] Many patients with primary hyperparathyroidism have vitamin D deficiency, and replacement may correct the elevated parathyroid hormone concentration. [48] There is also some weak evidence that calcium supplementation may decrease an isolated elevation in parathyroid hormone after parathyroidectomy. [46]
Quality of life has been shown repeatedly to be improved after parathyroidectomy. [49, 5] Notably, quality of life has also been found to undergo measurable improvement in “asymptomatic” patients, which underscores the fact that some mild symptoms may go unnoticed by patients and clinicians. [50]
A systematic literature review by Livschitz et al indicated that in patients with primary hyperparathyroidism, sustained improvement in quality of life can be achieved through parathyroidectomy. Quality of life was evaluated using such tools as the 36-Item Short Form Health Survey and the Parathyroidectomy Assessment of Symptoms score, with a median follow-up period of 1 year. In 27 out of 31 studies (87%), scores revealed significant, long-term improvement in quality of life, with the other reports showing mixed results. [51]
Follow-up
Patients are seen 1-2 weeks postoperatively, and serum calcium, 25-hydroxyvitamin D levels, and parathyroid hormone levels are obtained. Vitamin D deficiency is particularly common in patients with hyperparathyroidism. Many practitioners routinely add calcium and vitamin D supplementation postoperatively to help restore bone loss and supplement poor dietary intake.
Analysis has shown that the recurrence rate after successful parathyroidectomy is approximately 10-15% with long-term follow-up, which is much higher than had historically been thought. [52, 53] Long-term follow-up is therefore recommended, with annual calcium and parathyroid hormone determinations.
Secondary Hyperparathyroidism
Definition of problem
Secondary hyperparathyroidism is the overproduction of parathyroid hormone secondary to hypocalcemia, typically as a result of vitamin D deficiency and/or chronic kidney disease (CKD). [54]
Frequency
Vitamin D deficiency has been estimated to affect approximately 40% of US adults, according to data from the National Health and Nutrition Examination Survey (NHANES). [55] . Deficiency of the vitamin in adults has been attributed to decreases in sun exposure, changes in dietary intake, gastrointestinal disorders such as malabsorption syndromes and pancreatic insufficiency, and liver failure with decreased hydroxylation. In CKD, secondary hyperparathyroidism is common and varies based on estimated glomerular filtration rate (eGFR). In milder forms of CKD, elevations in parathyroid hormone levels occur in about 10% of patients, with the prevalence increasing to 90% of individuals with severe CKD who are approaching the need for dialysis therapy. [56]
Etiology
Secondary hyperparathyroidism results from a chronic stimulus of the parathyroid gland to release parathyroid hormone. Parathyroid hormone release is increased in the setting of hypocalcemia and hyperphosphatemia and is decreased by fibroblast growth factor 23 (FGF-23).
Parathyroid hormone acts on bone to release calcium into the blood and activates 1 alpha hydroxylation in the kidney to form 1,25-dihydroxvitamin D, which in turn increases calcium absorption in the gastrointestinal tract. The rise in calcium detected by the calcium-sensing receptor on the parathyroid gland reduces the stimulus for parathyroid hormone excretion.
Pathophysiology
Calcium and phosphorous homeostasis is tightly regulated between bone, the kidney, and the parathyroid gland. Key modulators of calcium and phosphorous include FGF-23, 25-hydroxyvitamin D, 1,25-dihydroxyvitamin D, and parathyroid hormone. FGF-23 is released from bone due to increasing serum phosphorus levels and acts in the kidney to increase phosphorous excretion and decrease 1 alpha hydroxylation of 25-hydroxyvitamin D. FGF-23, along with serum phosphorous, also decreases parathyroid hormone secretion, to maintain calcium and phosphorous balance. In CKD, stages 3-5 (eGFR < 59 mL/min), FGF-23 levels increase, initially leading to phosphaturia and decreased parathyroid hormone excretion. As the CKD progresses, there is a resistance in the kidney and parathyroid gland to FGF-23 and a deficiency in the kidney of 1 alpha hydroxylation of vitamin D, both of which contribute to reduced phosphorous excretion. The deficiency of 1,25-dihydroxyvitamin D, along with the decreased phosphorus excretion, results in hypocalcemia and hyperphosphatemia, thereby maintaining stimulation of parathyroid hormone synthesis and parathyroid gland hyperplasia.
Clinical presentation
Because virtually all patients with CKD have hyperparathyroidism to some degree, there is not a unique clinical presentation that is a hallmark of secondary hyperparathyroidism. Often, secondary hyperparathyroidism is discovered on routine laboratory testing when monitoring individuals with CKD. In patients with secondary hyperparathyroidism due to vitamin D deficiency, the symptoms result mainly from the vitamin deficiency (and include increased fracture risk in osteomalacia, and, rarely, myopathy). In advanced cases of secondary hyperparathyroidism, some patients may have bone pain.
Workup
Laboratory studies
The serum level of parathyroid hormone, calcium, phosphorus, and 25-hydroxyvitamin D should be measured. Patients with secondary hyperparathyroidism usually have low-normal calcium and elevated parathyroid hormone. The serum phosphorus level may vary based on the etiology, trending towards higher values with reduced kidney function and lower values with vitamin D deficiency.
Vitamin D deficiency is defined by most experts as a 25-hydroxyvitamin D level of less than 12 ng/mL. A level of 12-20 ng/mL may be considered insufficiency of vitamin D, and a level of 20 ng/mL or greater can be considered to indicate sufficient vitamin D. [57]
Imaging studies
Since all parathyroid glands will be affected in secondary hyperparathyroidism, localizing studies are not necessary, although they are increasingly being performed. Ultrasonography is noninvasive and may reveal thyroid pathology that was previously not recognized and that should be dealt with at the time of neck exploration. Bone density may be determined to assess the severity of bone disease. Radiographs of sites of bone pain are reasonable but seldom necessary. Hand radiographs may show characteristic subperiosteal erosions.
Treatment
Medical care
Unlike primary hyperparathyroidism, medical management is the mainstay of treatment for secondary hyperparathyroidism.
Correcting vitamin D deficiency may be achieved using cholecalciferol (vitamin D3) or ergocalciferol (vitamin D2). Recommended therapy for patients with vitamin D deficiency is 50,000 IU of vitamin D2 or D3 once a week for 8 weeks and then supplementation with 800 IU daily indefinitely.
For patients with CKD, the National Kidney Foundation (NKF) published clinical practice guidelines as part of its Kidney Disease Outcomes Quality Initiative (KDOQI). In general, it has been recommended to reduce parathyroid hormone levels to within a range that supports normal bone turnover and minimizes ectopic calcification.
The following is a list of current nonsurgical treatment options for management of secondary hyperparathyroidism in CKD:
-
Dietary phosphorus restriction may be initiated if parathyroid hormone is elevated despite sufficient 25-hydroxyvitamin D (>20 ng/mL).
-
Phosphate binders may be considered if hyperphosphatemia persists despite dietary phosphate restriction. These include calcium-based phosphate binders such as calcium carbonate or calcium acetate and non–calcium-based phosphate binders such as sevelamer hydrochloride, lanthanum carbonate, ferric citrate, or sucroferric oxyhydroxide.
-
Non–calcium-based binders are generally preferred for use over calcium-based binders due to concern that excess calcium supplementation may increase coronary artery calcification and cardiovascular risk. [58]
-
Vitamin D and its analogs may be used—including calcitriol and analogs of calcitriol such as calcifediol, paricalcitol, doxercalciferol, maxacalcitol, and falecalcitriol—to treat hypocalcemia and secondary hyperparathyroidism. [59] Treatment with calcimimetics such as cinacalcet leads to significant improvements in biochemical parameters, but patient-based benefits have not yet been demonstrated.
-
The newest class of drugs to reduce the parathyroid hormone level, the aforementioned calcimimetics—which includes etelcalcetide (Parsabiv) and cinacalcet (Sensipar)—binds to and activates the calcium-sensing receptor. Activation of this receptor on parathyroid chief cells decreases parathyroid hormone secretion. Etelcalcetide can be administered intravenously at the end of hemodialysis sessions. Its approval was based on randomized, double-blind trials that compared the drug to placebo and cinacalcet. Patients in both studies were on hemodialysis and had moderate to severe secondary hyperparathyroidism. Those taking etelcalcetide had a significantly greater chance of reaching the primary efficacy endpoint (>30% reduction from baseline in mean predialysis parathyroid concentration at 20-27 weeks) than did patients receiving placebo (74-75.3% vs 8.3-9.6%, respectively). [54] When compared with cinacalcet, etelcalcetide was superior in decreasing parathyroid hormone levels over the span of 26 weeks. [60]
-
The EVOLVE (EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events) trial was one of the first large-scale, randomized controlled trials in patients with ESRD to examine the treatment of secondary hyperparathyroidism with cinacalcet vs placebo along with standard of care phosphorous binders and vitamin D analogs. Hyperphosphatemia and hyperparathyroidism have been associated with increased risk of death in ESRD patients and this trial was designed to see if there was a mortality benefit in the use of the noncalcium/non–vitamin D analog cinacalcet in the ESRD population. The results of the trial demonstrated that cinacalcet did not result in improved mortality or improve cardiovascular endpoints. The use of cinacalcet did reduce the need for parathyroidectomy for refractory hyperparathyroidism. Of note, there was an increased risk of serious adverse events, namely hypocalcemia, with the use of cinacalcet. In conclusion, despite aggressive management of secondary hyperparathyroidism that is associated with increased mortality in ESRD patients, cinacalcet did not offer a cardiovascular or mortality benefit in this population of patients. [61]
Surgical care
Indications for surgery include bone pain or fracture, pruritus, calciphylaxis (see Related Disorders), and extraskeletal nonvascular calcifications with elevated parathyroid hormone levels despite appropriate medical therapy. Parathyroidectomy can be considered in patients with severe hyperparathyroidism (persistent serum levels of intact parathyroid hormone greater than 800 pg/mL [88.0pmol/L]), associated with hypercalcemia and/or hyperphosphatemia that is refractory to medical therapy.
A study by Hsu et al indicated that in patients with severe secondary hyperparathyroidism resulting from end-stage renal disease, parathyroidectomy reduces the risk of peripheral arterial disease. In the end-stage renal disease patients who underwent parathyroidectomy (947 patients, 5.08-year follow-up), the incidence density rate of peripheral arterial disease was 12.26 per 1000 person-years, compared with 24.09 per 1000 person-years in those who did not undergo the surgery (3746 patients, 4.52-year follow-up). [62]
Mortality has been shown to be reduced in dialysis patients who undergo parathyroidectomy for severe secondary hyperparathyroidism.
Surgical treatment of severe secondary hyperparathyroidism may also improve quality of life. In a meta-analysis of patients with secondary hyperparathyroidism treated with cinacalcet or parathyroidectomy, quality of life was seen to improve after surgical treatment but not with medical therapy. [63] However, available studies into quality of life in such cases are observational, and randomized, comparative data are not available.
Surgical technique
The general surgical technique in secondary hyperparathyroidism involves complete parathyroid exploration, as described previously for primary hyperparathyroidism. All four glands must be exposed, and biopsies are taken if needed to ensure correct identification. In most cases, diffuse hyperplasia is encountered, although the size of the glands can be significantly heterogeneous.
The treatment procedure of choice is either total parathyroidectomy with autotransplantation or subtotal parathyroidectomy. In a study by Rothmund et al, a randomized, controlled trial of total versus subtotal parathyroidectomy for secondary hyperparathyroidism, the investigators found that four of 17 subjects treated with subtotal parathyroidectomy developed recurrent hypercalcemia, with two requiring reexploration. None of the subjects treated with total parathyroidectomy developed recurrent hypercalcemia. [64] A subtotal parathyroidectomy has the advantage of less dramatic postoperative hypocalcemia. However, reoperative neck exploration is required in the case of recurrence, which can be very difficult. Another approach is to perform total parathyroidectomy without autotransplantation. Good results have been reported, but at present this procedure should be considered investigational and should be reserved only for patients in whom future transplantation is not an option. [65, 66, 67]
Parathyroid autotransplantation is usually performed after a total parathyroidectomy. Briefly, about 100 mg of parathyroid tissue is cut into approximately 12-20 pieces, each of which measures about 1 x 1 mm. These are inserted into pockets in the forearm, either in the subcutaneous tissue or the musculature (the forearm being chosen primarily for convenience). After surgery, blood draws from the antecubital fossa above the transplantation site can be compared with blood drawn from the contralateral side to assess graft function. The site may be marked with a polypropylene suture on the fascia for localization later, if necessary. We also use clips to facilitate localization with ultrasonography. Parathyroid tissue may also be cryopreserved in case the primary autotransplant fails.
Most patients require admission after subtotal parathyroidectomy or total parathyroidectomy with autotransplantation. Hypocalcemia is to be expected and is often severe, especially in persons who have had a history of very high parathyroid hormone levels for many years. Although hypocalcemia is usually more severe after total parathyroidectomy with autotransplantation, it can occur after subtotal parathyroidectomy as well. A constant calcium infusion is often required, and oral calcium and calcitriol requirements can be quite high. The target calcium level should typically be below the normal range but above the level at which the patient is symptomatic. The patient can be discharged when he or she is able to maintain a safe calcium level on oral supplements. Typically, the patient can be weaned off of these over a period of weeks to a few months.
Outcome and prognosis
Medical treatment of secondary hyperparathyroidism is successful in most patients. Patients who require parathyroidectomy have approximately a 10% risk of recurrent or persistent disease. This may be due to a hyperfunctioning or missed neck gland or hyperplasia of the autograft. Occasionally, a patient has persistent hypoparathyroidism after operation. If tissue has been cryopreserved, transplantation may reverse hypoparathyroidism. If hypoparathyroidism is permanent, lifelong calcium and calcitriol supplementation is necessary.
Tertiary Hyperparathyroidism
Definition of problem
Tertiary hyperparathyroidism is a state of excessive secretion of parathyroid hormone after longstanding secondary hyperparathyroidism and resulting in hypercalcemia. Some authorities reserve the term for secondary hyperparathyroidism that persists after successful renal transplantation.
Etiology
Tertiary disease is characterized by the development of autonomous hypersecretion of parathyroid hormone causing hypercalcemia. The etiology is unknown but may be due to monoclonal expansion of parathyroid cells (nodule formation within hyperplastic glands). A change may occur in the set point of the calcium-sensing mechanism to hypercalcemic levels. Four-gland involvement occurs in most patients.
Pathophysiology
Tertiary hyperparathyroidism is observed most commonly in patients with chronic secondary hyperparathyroidism who have been on dialysis therapy for years. The hypertrophied parathyroid glands enlarge over time and continue to oversecrete parathyroid hormone, despite serum calcium levels that are within the reference range or even elevated. In these cases, the hypertrophied glands become autonomic and cause hypercalcemia, even after withdrawal of calcium and active vitamin D therapy. They also may become resistant to calcimimetic treatment. [68, 69] This type of tertiary disease is particularly dangerous because the phosphate level is often elevated. If the calcium value multiplied by the phosphate value yields a high product, diffuse calcinosis may occur.
Clinical presentation
The clinical manifestations of tertiary hyperparathyroidism are related to the effects of hypercalcemia and hyperphosphatemia, including bone pain, pruritus, fatigue, lethargy, and increased risk for fractures.
Treatment
Total parathyroidectomy with autotransplantation or subtotal parathyroidectomy is indicated.
Related Disorders
Familial benign (hypocalciuric) hypercalcemia
Familial benign (hypocalciuric) hypercalcemia (FHH) is caused by a loss-of-function mutation of one allele of the gene for the calcium-sensing receptor (CaR). It causes hypercalcemia, hypophosphatemia, and hypermagnesemia. The parathyroid hormone level is usually within the reference range or is mildly elevated. It can be distinguished from primary hyperparathyroidism by low 24-hour urinary calcium excretion. Persons with FHH are asymptomatic. Parathyroidectomy is not indicated.
Hypercalcemia of malignancy
This disorder is usually caused by tumor release of a hormone called parathyroid hormone -related peptide. Less commonly, hypercalcemia of malignancy is caused by local osteolytic lesions and, rarely, by overproduction of 1,25-dihydroxyvitamin D. This disorder is the most common cause of hypercalcemia in hospitalized patients. The hypercalcemia of malignancy results in a low or undetectable intact parathyroid hormone level. Usually, it is easily distinguished from hyperparathyroidism. Only a few cases of ectopic production of true parathyroid hormone are reported in the literature.
Calciphylaxis
Calciphylaxis, also known as uremic gangrene syndrome, is a rare disorder observed in patients with CKD, typically ESRD, and secondary or tertiary hyperparathyroidism. It is characterized by ischemic necrosis of the skin due to calcium phosphate crystal deposition and subsequent inflammation in small-to-medium–sized vessels. The exact mechanism is unknown because the product of the calcium value multiplied by the phosphate value is often near the reference range. The disease is often fatal. There is some evidence that the use of sodium thiosulfate, a chelator of calcium and iron, may improve wound healing and outcomes in patients with calciphylaxis. [70] Total parathyroidectomy may also reverse the necrosis and is occasionally done emergently.
Questions & Answers
Overview
What anatomy is relevant in hyperparathyroidism?
What is primary hyperparathyroidism?
What is the prevalence of primary hyperparathyroidism in the US?
What causes primary hyperparathyroidism?
What is the pathophysiology of primary hyperparathyroidism?
What are the signs and symptoms of primary hyperparathyroidism?
Which physical findings are characteristic of primary hyperparathyroidism?
What causes hypercalcemia in primary hyperparathyroidism?
What is the role of lab studies in the evaluation of primary hyperparathyroidism?
What is the role of imaging studies in the workup of primary hyperparathyroidism?
What is the role of ultrasonography in the workup of primary hyperparathyroidism?
What is the role of nuclear scanning in the workup of primary hyperparathyroidism?
What is the role of CT scanning and MRI in the workup of primary hyperparathyroidism?
Which patients should undergo surgery for primary hyperparathyroidism?
According to guidelines, when is surgery indicated for primary hyperparathyroidism?
How is asymptomatic primary hyperparathyroidism managed?
How is severe hypercalcemia managed in primary hyperparathyroidism?
What long-term monitoring is needed for asymptomatic primary hyperparathyroidism?
Which medications are used in the treatment of primary hyperparathyroidism?
What is the role of percutaneous ablation in the treatment of primary hyperparathyroidism?
What is the role of directed parathyroidectomy in the treatment of primary hyperparathyroidism?
How are multiple abnormal glands detected in primary hyperparathyroidism?
Why do best practices needed for parathyroidectomy in the treatment of primary hyperparathyroidism?
What is the role of parathyroidectomy in the treatment of primary hyperparathyroidism?
How is a directed parathyroidectomy performed for the treatment of primary hyperparathyroidism?
What long-term monitoring is needed following treatment for primary hyperparathyroidism?
What is the prevalence of secondary hyperparathyroidism?
What is secondary hyperparathyroidism?
What causes secondary hyperparathyroidism?
What is the pathophysiology of secondary hyperparathyroidism?
What are the signs and symptoms of secondary hyperparathyroidism?
What is the role of lab studies in the workup of secondary hyperparathyroidism?
What is the role of imaging studies in the workup of secondary hyperparathyroidism?
How is secondary hyperparathyroidism managed?
What are the nonsurgical treatment options for secondary hyperparathyroidism?
What is the role of surgery in the treatment of secondary hyperparathyroidism?
Which surgical procedures are performed in the treatment of secondary hyperparathyroidism?
What is the prognosis of secondary hyperparathyroidism?
What is tertiary hyperparathyroidism?
What causes tertiary hyperparathyroidism?
What is the pathophysiology of tertiary hyperparathyroidism?
What are the signs and symptoms of tertiary hyperparathyroidism?
What is the treatment of tertiary hyperparathyroidism?
How is familial benign (hypocalciuric) hypercalcemia differentiated from hyperparathyroidism?
How is hypercalcemia of malignancy differentiated from hyperparathyroidism?
-
Normal parathyroid glands as seen during a thyroidectomy. The large arrow points to the superior parathyroid. The thinner arrow points to the inferior parathyroid. The forceps points toward the recurrent laryngeal nerve. The patient's head is toward the top right.
-
Hyperparathyroidism. Technetium-99m (99mTc) sestamibi radionuclide scan. The early image (top left) shows uptake in the salivary glands and thyroid. The later images (right and bottom) show washout from the thyroid but persistence in the region of the right inferior thyroid lobe (arrows). This proved to be a right parathyroid adenoma.
-
Sestamibi parathyroid scan with SPECT scan. The orange indicates radionuclide accumulation. The findings indicate the presence of a right-sided parathyroid adenoma just behind the thyroid lobe. At exploration, this patient was found to have adjacent double adenomas on the right.
-
Sestamibi parathyroid scan with SPECT scan showing an ectopic mediastinal parathyroid adenoma adjacent to the aortic arch. This patient had undergone a failed neck exploration. A transthoracic, robotic excision was curative.
-
A small, left inferior parathyroid adenoma as demonstrated on a 4D-CT scan. The left panel is a single image from the early contrast phase showing intense enhancement. The right panel shows rapid washout of contrast. The white arrows point to the adenoma.
-
The panels show a transverse and longitudinal (sagittal) view of a left superior parathyroid adenoma on ultrasonography. The caliper marks designate the adenoma. Adenomas are typically homogeneous and hypoechoic.