Practice Essentials
Community-acquired pneumonia (CAP) is one of the most common infectious diseases and an important cause of mortality and morbidity worldwide. Typical bacterial pathogens that cause CAP include Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. However, with the advent of novel diagnostic technologies, viral respiratory pathogens are increasingly being identified as frequent etiologies of CAP. The most common viral pathogens recovered from hospitalized patients admitted with CAP include human rhinovirus and influenza. [1]
Presentation and pathogens in typical community-acquired pneumonia
The term “typical” CAP refers to a bacterial pneumonia caused by pathogens such as S pneumoniae, H influenzae, and M catarrhalis. Patients with typical CAP classically present with fever, a productive cough with purulent sputum, dyspnea, and pleuritic chest pain. Characteristic pulmonary findings on physical examination include the following:
-
Tachypnea
-
Rales heard over the involved lobe or segment
-
Increased tactile fremitus, bronchial breath sounds, and egophony may be present if consolidation has occurred.
-
Decreased tactile fremitus and dullness on chest percussion may result from parapneumonic effusion or empyema.
Epidemiologic data may provide clues to the specific pathogen causing CAP, as follows:
-
The most common bacterial pathogen overall is S pneumoniae, although, in some settings, including in the United States, its incidence is decreasing, possibly owing to vaccination. [1]
-
Underlying chronic obstructive pulmonary disease (COPD) [2] : H influenzae or M catarrhalis
-
Alcoholic patient presenting with “currant jelly” sputum : Klebsiella pneumoniae [2]
Atypical community-acquired pneumonia
The clinical presentation of so-called “atypical” CAP often is subacute and frequently is indolent. In addition, patients with atypical CAP may present with more subtle pulmonary findings, nonlobar infiltrates on radiography, and various extrapulmonary manifestations (eg, diarrhea, otalgia). Atypical CAP pathogens include the following:
-
Mycoplasma pneumoniae
-
Chlamydophila ( Chlamydia) pneumoniae
-
Legionella pneumophila (Legionnaires disease)
-
Respiratory viruses, including the following:
- SARS-CoV-2 (COVID-19)
- Influenza A and B
- Rhinovirus
- Respiratory syncytial virus
- Human metapneumovirus
- Adenovirus 4 and 7
- Parainfluenza virus
-
Other rare CAP pathogens:
- Viruses
- Coxsackievirus
- Echovirus
- Other coronaviruses (MERS-CoV, SARS)
- Hantavirus
- Epstein-Barr virus
- Cytomegalovirus
- Herpes simplex virus
- Human herpesvirus 6
- Varicella-zoster virus
- Metapneumovirus [5]
- Bacteria
- Chlamydophila psittaci (psittacosis)
- Coxiella burnetii (Q fever)
- Francisella tularensis (tularemia)
- Mycobacteria
- Mycobacterium tuberculosis
- Nontuberculous mycobacteria (uncommon)
- Endemic fungi (causing subacute or chronic pneumonia)
- Histoplasma capsulatum
- Cryptococcus neoformans neoformans and neoformans gattii
- Coccidioides immitis
- Viruses
Extrapulmonary signs and symptoms seen in some forms of atypical CAP may include the following:
-
Mental confusion
-
Prominent headaches
-
Myalgias
-
Ear pain
-
Abdominal pain
-
Diarrhea
-
Rash (Horder spots in psittacosis; erythema multiforme in Mycoplasma pneumonia)
-
Nonexudative pharyngitis
-
Hemoptysis
-
Splenomegaly
-
Relative bradycardia
Historical clues and physical examination findings may suggest a causative pathogen, but the clinical signs and symptoms of CAP are not sufficiently specific to reliably differentiate the exact etiologic agent. [6] Therefore, additional testing remains necessary to identify the pathogen and to optimize therapy in CAP.
Workup
Standard diagnostic studies for CAP include the following:
-
Chest radiography
-
Complete blood cell (CBC) count with differential
-
Serum blood urea nitrogen (BUN) and creatinine levels
For patients with severe CAP, patients being empirically treated for methicillin-resistant S aureus (MRSA) or Pseudomonas, or patients in whom a specific etiology is suspected, additional workup may be warranted, including the following [7] :
-
Sputum Gram stain and/or culture
-
Blood cultures
-
Serum sodium level
-
Serum transaminase levels
-
Lactic acid level
-
C-reactive protein (CRP)
-
Lactate dehydrogenase (LDH)
-
Molecular diagnostics, ie, polymerase chain reaction (PCR) testing
-
Urinary antigen testing for Legionella species
In special situations:
-
During influenza season, molecular assay for influenza
-
Molecular or antigen testing for SARS-CoV-2 with ongoing community transmission
-
Serologic studies for M pneumoniae, C pneumoniae, Bordetella pertussis, C burnetii
-
Creatine phosphokinase (CPK)
-
Serum phosphorus level
Chest radiography
Obtain chest radiographs in all patients with suspected CAP to evaluate for an infiltrate and to help exclude conditions that may mimic CAP, such as lung cancer or pulmonary emboli. [8, 9] Patients who present very early with CAP may have negative findings on chest radiography. In these patients, repeat chest radiography within 24 hours may be beneficial. CT scanning may also be necessary in immunocompromised patients who present with symptoms that suggest CAP or ambiguous symptoms in whom chest radiography findings are negative. Serial chest radiography may be used to observe the progression of CAP; however, radiographic improvement often lags behind clinical improvement.
Hospital admission
Multiple scoring systems are available to assess the severity of CAP and to assist in deciding whether a patient should be hospitalized or admitted to the intensive care unit (ICU). Severe pneumonia is defined as having one major or three minor criteria.
Major criteria include [7] :
-
Septic shock requiring vasopressors
-
Respiratory failure requiring mechanical ventilation.
Minor criteria include the following [7] :
-
Respiratory rate of 30 or more breaths per minute
-
PaO 2/FIO 2 ratio of 250 or less
-
Multilobar infiltrates
-
Confusion
-
Uremia
-
Leukopenia (WBC < 4000 cells/µl)
-
Thrombocytopenia (platelet count < 100,000/µl)
-
Hypothermia
-
Hypotension
The Pneumonia Severity Index (PSI) is preferred over the CURB-65 (confusion, uremia, respiratory rate, low blood pressure, age >65 years) for determining outpatient versus inpatient treatment. Patients with PSI class IV-V may need hospitalization or more intensive in-home services. ICU admission is recommended for any patient who requires mechanical ventilation or vasopressors. Admission to higher-acuity care or critical care should also be considered in patients with three or more minor risk factors for severe pneumonia. Even in the setting of COVID-19, PSI was still shown to be more predictive of mortality than CURB-65. [10]
Other scoring systems may also be helpful in certain populations to predict the severity of CAP. The SMART-COP score emphasizes the ability to predict the need for ventilator or vasopressor support and includes systolic blood pressure, multilobar infiltrates, serum albumin levels, respiratory rate, tachycardia, confusion, oxygenation, and pH level. The A-DROP (age, dehydration, respiratory failure, orientation, systolic blood pressure) is also a severity score. Recently, an expanded CURB-65 has been shown to improve prediction of 30-day mortality. It includes LDH, thrombocytopenia, and serum albumin, along with the traditional CURB-65, and has been shown to have better prediction accuracy. The value of adding biomarkers in addition to the above scoring systems to identify patients at risk of worse outcomes is being studied. [11, 12, 13, 14, 15]
More recent research indicates that the use of biomarkers, specifically procalcitonin and CRP, may further increase the ability to identify patients at increased risk for worse outcomes, although this remains controversial. A positive pneumococcal urine antigen result has been associated with a good prognosis. [16] Certain prior pulmonary pathology, such as past infection with pulmonary tuberculosis, may increase the risk of mortality. [17]
Antibiotic Therapy
Adequate empiric antimicrobial therapy for CAP includes coverage for S pneumoniae and atypical bacterial pathogens. Outpatient treatment for CAP in patients with no comorbidities and no risk factors for drug-resistant S pneumoniae frequently includes the following [7] :
-
Amoxicillin 1 g PO three times a day OR
-
A macrolide (azithromycin 500 mg once and then 250 mg daily, clarithromycin 500 mg twice daily) OR
-
Doxycycline 100 mg twice daily
Macrolides should be used only in areas where pneumococcal resistance is less than 25%. During influenza season, it is also reasonable to initiate oseltamivir, zanamivir, peramivir, or baloxavir therapy in outpatients who present with a flulike illness and pneumonia.
Treatment options for CAP in patients with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; asplenia; immunosuppression; prior antibiotics within 90 days; or other risk factors for drug-resistant infection include the following:
-
Beta-lactam ( amoxicillin/clavulanate 2 g/125 mg twice daily or 500 mg/125 mg three times daily or 875 mg/125 mg twice daily, cefpodoxime 200 mg twice daily, or cefuroxime 500 mg twice daily) AND a macrolide or doxycycline OR
-
Respiratory fluoroquinolones ( moxifloxacin 400 mg daily, levofloxacin 750 mg daily)
For hospitalized patients, therapy consists of the following:
-
Beta-lactams (ampicillin/sulbactam 1.5-3 g every 6 hours, ceftriaxone 1-2 g daily, cefotaxime 1-2 g every 8 hours, or ceftaroline 600 mg every 12 hours) AND a macrolide OR
-
Respiratory fluoroquinolone OR
-
If macrolides and fluoroquinolones are contraindicated: Beta-lactam as above AND doxycycline
Therapy in ICU patients includes the following:
-
Beta-lactam (ceftriaxone, cefotaxime, or ampicillin/sulbactam) AND either a macrolide or respiratory fluoroquinolone
-
For patients with penicillin allergy, a respiratory fluoroquinolone AND aztreonam
Risk factors for Pseudomonas pneumonia include structural lung disease, COPD, and bronchiectasis [4] If Pseudomonas is suspected, therapy is as follows:
-
Anti-pneumococcal and anti-pseudomonal beta-lactam (piperacillin/tazobactam 4.5 g every 6 hours, cefepime 2 g every 8 hours, ceftazidime 2 g every 8 hours, meropenem 1 g every 8 hours, or imipenem 500 mg every 6 hours). [7]
-
In patients with severe penicillin allergy, aztreonam 2 g every 8 hours may be used instead of the beta-lactam in the regimen listed above. It is worth noting that many reported penicillin allergies are not true allergies. Owing to the limited spectrum of aztreonam and the relatively low likelihood of penicillin allergy cross-reacting with cephalosporins (2%), cefepime is a reasonable choice after considering the balance of benefit and risk. [18]
If methicillin-resistant S aureus (MRSA) is suspected, vancomycin 15 mg/kg every 12 hours adjusted based on levels or linezolid 600 mg every 12 hours should be added. Risk factors for MRSA include hemoptysis, recent influenza, neutropenia, hemodialysis, and congestive heart failure. [4] During influenza season, it is also reasonable to start oseltamivir, zanamivir, peramivir, or baloxavir therapy to treat influenza in patients with MRSA CAP, as well as in patients who present with a flulike illness and pneumonia, as influenza may have preceded the MRSA infection. However, oseltamivir remains the preferred agent in inpatients with suspected or confirmed influenza, as there is limited data to support the alternative antivirals in severe or complicated disease. [19]
Rapid initiation of therapy is important for improved outcomes in CAP, although blanket measures to hasten treatment are not without potential negative consequences. Quality-improvement efforts aimed at the administration of antibiotics within a certain time period have contributed to increased inappropriate antibiotic use and increased incidence of Clostridium difficile colitis. Nevertheless, in patients with signs of severe CAP or sepsis, antibiotics should be given within the first hour of hypotension onset to reduce mortality. [20] At minimum, blood cultures and, ideally, sputum cultures should be collected prior to the first antibiotic dose, although antibiotics should not be delayed. However, recent studies have demonstrated that imposing a strict universal time to first antibiotic dose may not be as beneficial in CAP, instead predisposing to excess antibiotic use. Using clinical indicators suggestive of more severe disease may be more beneficial in determining who needs rapid antibiotic administration, such as advanced age, increasing medical comorbidities, or need for supplemental oxygen upon admission. [21]
Results of respiratory specimen cultures, blood cultures, and pleural fluid analysis; PCR of respiratory samples; or antigen tests should be monitored and used to target therapy whenever possible. Inpatient CAP therapy usually consists of intravenous antibiotics followed by transition to an oral course of therapy. [22, 23, 24, 25] Patients who are severely ill or who are unable to tolerate or absorb oral medications may require a longer duration of parenteral therapy before switching to an oral antibiotic. [26]
Mild to moderately ill patients with CAP may be treated entirely via the oral route, on either an inpatient or outpatient basis. The duration of therapy for uncomplicated CAP is usually 5 days. [27, 20] The duration of therapy for CAP due to suspected or proven Pseudomonas or MRSA is typically 7 days. Patients should be afebrile for 48 to 72 hours and have no signs of instability before antibiotic therapy is stopped. The duration of therapy may need to be increased if the initial empiric therapy has no activity against the specific pathogen or if the pneumonia is complicated by extrapulmonary infection. [7] Immunocompromised hosts who present with CAP are treated in a manner similar to that of otherwise healthy hosts but may require a longer duration of therapy. Additional investigations into pathogens associated with immunocompromised hosts may also need to be pursued.
Overview
Community-acquired pneumonia (CAP) is one of the most common infectious diseases addressed by clinicians and is an important cause of mortality and morbidity worldwide.
Typical bacterial pathogens that cause CAP include S pneumoniae, H influenzae, and M catarrhalis. Numerous other organisms can cause CAP in the appropriate clinical setting. Furthermore, the so-called “atypical CAP” pathogens are actually common causes of CAP and were originally classified as atypical because they are not readily detectable on Gram stain or cultivatable on standard bacteriologic media.
CAP is usually acquired via inhalation or aspiration of a pathogenic organism.
Aspiration pneumonia is commonly caused by multiple pathogens (eg, aerobic/anaerobic oral organisms).
Severe community-acquired pneumonia
Severe CAP frequently develops in individuals with comorbid factors such as underlying cardiopulmonary disease, diminished splenic function, and/or heightened pathogenic virulence. Even in young and/or healthy hosts, severe CAP can develop if the causative pathogen is sufficiently virulent. For example, influenza, severe acute respiratory syndrome (SARS), Coronavirus disease 2019 (COVID-19), Hantavirus pulmonary syndrome (HPS), and Legionnaires disease may present as severe CAP. [28, 29, 30, 31, 32]
Patients with severe CAP should have the benefit of an infectious disease specialist to assist in identifying and optimally managing the underlying cause.
Complications associated with community-acquired pneumonia
The risk for complications in CAP depends on the infecting pathogen and the patient’s baseline health. For example, various organisms can cause empyema, including S pneumoniae, K pneumoniae (classically occurring in patients with chronic alcoholism), group A Streptococcus, and S aureus. Cavitation is not a typical feature of pneumococcal pneumonia but is relatively common in K pneumoniae infections.
Cardiovascular complications, including acute myocardial infarction or congestive heart failure (CHF), may be precipitated by CAP in up to one third of patients. [33, 34, 35] A 2019 post hoc analysis of a cluster randomized controlled trial indicated increased rates of hospital-acquired cardiac events, mostly heart failure, after erythromycin use. [36] Viral pneumonia such as COVID-19 has also been associated with increased rates of myocarditis. [37]
Patients with CAP who have impaired splenic function may develop overwhelming pneumococcal sepsis, potentially leading to death within 12 to 24 hours, regardless of the antimicrobial regimen used.
Morbidity and mortality
Morbidity and mortality associated with CAP are most common in elderly patients and immunocompromised hosts.
Other factors that may portend an increased risk for morbidity and mortality in individuals with CAP include significant comorbidities such as structural lung disease or cancer, and presenting signs of severe infection such as an increased respiratory rate, hypotension, fever, multilobar involvement, anemia, and hypoxia. [38] An elevated procalcitonin level may also be associated with an increased mortality risk, although the specific threshold for increased risk remains to be determined. [13]
For more information, see the following:
Etiology of Community-Acquired Pneumonia
The definitive microbiologic etiology is determined in less than half of patients who develop community-acquired pneumonia (CAP); the exact rate depends on the patient population and diagnostic testing used. [1, 39, 40] Organisms traditionally have been classified as “typical” or “atypical” CAP pathogens, depending on their ability to be detected on Gram stain or standard bacterial cultures.
Typical community-acquired pneumonia pathogens
Typical bacterial pathogens that cause CAP include S pneumoniae, H influenza, and M catarrhalis (Gram stains shown below). The frequency with which CAP is attributable to one of these pathogens varies according to epidemiologic factors (eg, seasonality, patient demographics, exposure history) and the diagnostic testing used. In the past, these organisms had been reported to account for most CAP cases. [41] However, with improvement in diagnostic techniques allowing for better identification of viruses and fastidious bacteria, our understanding of the etiologic agents involved in the development of CAP has evolved. As a result, a smaller percentage of CAP cases are now being attributed to these typical bacterial pathogens. [42] In addition, the possibility of polymicrobial infections has received increased awareness; in recent epidemiologic studies, two pathogens, typically a virus and bacteria combination, were identified in over 30% of cases. [1, 39, 5]
S pneumoniae remains the most common bacterial agent responsible for CAP. The incidence of S pneumoniae pneumonia varies according to the population studied. A 2015 study of 267 patients with CAP in Norway reported that S pneumoniae accounted for 30% of cases; this represented 48.5% of the cases in which an organism was identified. [39] A separate study of adults with CAP in the United States identified S pneumoniae as the etiologic agent in only 5% of total cases of CAP (13.5% of cases with an identified pathogen). [1] Declining tobacco smoking and widespread pneumococcal vaccination have been postulated as potential contributors to the decreased rates of S pneumoniae infection in the United States. [40, 42]
S aureus traditionally has not been considered a typical cause of CAP in otherwise healthy hosts, with the exception of potentially severe CAP after influenza infection. [3] Community-acquired methicillin-resistant S aureus (MRSA) has been associated with multilobar necrotizing CAP, including in previously healthy individuals. However, in a prospective multicenter US surveillance study of 2259 adults hospitalized for CAP, S aureus was identified in only 1.6% of patients, and MRSA accounted for only 0.7% of all cases. [43] Interestingly, in that study, the clinical presentation of MRSA CAP did not differ from that of all-cause non–S aureus or pneumococcal CAP with regard to concurrent influenza infection, presence of multilobar infiltrates, or hemoptysis. MRSA CAP was significantly associated with prior long-term hemodialysis use and carried a higher inpatient mortality rate (13.3%) than all-cause non–S aureus CAP (2%). [43]
Importantly, K pneumoniae and Pseudomonas aeruginosa are not typical causes of CAP in otherwise healthy hosts. K pneumoniae CAP occurs primarily in individuals with chronic alcoholism or diabetes mellitus. P aeruginosa is a cause of CAP in patients with underlying lung disease such as bronchiectasis or cystic fibrosis.
In certain patients admitted to the ICU, the microbial etiology of pneumonia may be complex. In a study by Cilloniz et al, 11% of cases were polymicrobial. The most frequently identified pathogens in polymicrobial infections were S pneumoniae, respiratory viruses, and P aeruginosa. Chronic respiratory disease and acute respiratory distress syndrome (ARDS) criteria were independent predictors of a polymicrobial infection. [44] Bacterial and viral co-infection may portend a worse prognosis. [45, 46]
Other gram-negative pathogens (eg, Enterobacter species, Serratia species, Stenotrophomonas maltophilia, Burkholderia cepacia) rarely cause CAP in patients without underlying lung disease or immunosuppression.
Atypical community-acquired pneumonia pathogens
Atypical bacterial pneumonias can be differentiated into those caused by zoonotic or nonzoonotic atypical pathogens.
Zoonotic atypical CAP pathogens include Chlamydophila (Chlamydia) psittaci (psittacosis), F tularensis (tularemia), and C burnetii (Q fever).
Nonzoonotic atypical CAP pathogens include Legionella species (Legionnaires disease), M pneumoniae, and C pneumoniae. [47]
Respiratory viruses are another important cause of atypical CAP. While certain viruses may be zoonotically transmitted (eg, Hantavirus and avian influenza), most are transmitted from person to person.
Epidemiology of Community-Acquired Pneumonia
The incidence of CAP varies greatly from country to country. In the United States, CAP is estimated to occur in 248 of 10,000 adults annually, whereas a study from the Veterans Health Administration noted 472.2 cases per 100,000 person-years in 2017. [48] Other countries report different frequencies, such as 8.1 per 10,000 in Vietnam and 31.2 per 10,000 in the United Kingdom. Rates also vary based on the population studied. In adults older than 65 years, 130.5 per 10,000 develop CAP in Malaysia, whereas 76.5 per 10,000 develop CAP in Germany. In individuals aged 85 years or older in Norway, the incidence of CAP is 172.4 per 10,000. [5] Influenza and pneumonia combined remain in the top 10 leading causes of death in the United States, ranking 9 of 10 in 2020. The combination was responsible for 1.6% (53,495) of deaths in 2020, an absolute increase of 7.5% from 2019 but a relative decrease due to the separately accounted deaths due to COVID-19, which ranked 3 of 10 at 10.3% (345,323) of all deaths. [49] In addition, survivors of CAP have an increased incidence of cardiovascular events and mortality risk for months to years after CAP. As many as 20% of CAP survivors have been shown to have cardiovascular events, and mortality is up to 30% 2 to 5 years after CAP. [5]
The cost of CAP has been examined in numerous studies. In a 2018 retrospective analysis of claims data for adults aged 65 years or older in a Medicare insurance plan, the rate of CAP was 846.7 per 100,000 person-years, which was greater than rates for myocardial infarction (405), stroke (278.9), and osteoporotic fractures (343.9). This study noted vaccinations against influenza and Pneumococcus infection cost $40 million; however, prevention for stroke and myocardial infarction cost more than $661 million. [50]
Age
Advanced age is associated not only with a higher incidence of CAP but also with more severe disease, greater need for hospitalization, and higher mortality. [27, 51] A recent US study estimated 967,470 adults aged 65 and over are hospitalized annually from CAP with a 38% one-year mortality. [52]
CAP encountered in the ambulatory setting is more common among young adults, and is usually due to so-called atypical CAP pathogens (eg, Mycoplasma pneumoniae). [53]
Extrapulmonary Findings in Atypical Community-Acquired Pneumonia
Atypical community-acquired pneumonia (CAP) has classically been associated with more extrapulmonary manifestations than typical bacterial CAP. However, there can be considerable overlap in the clinical presentation of CAP due to various pathogens such that a definitive microbiologic diagnosis cannot be made based on signs and symptoms alone. Certain constellations of findings in the setting of appropriate historical clues may suggest an increased likelihood of a specific pathogen, thus warranting a targeted investigation for that organism. A detailed review of all potential extrapulmonary findings in CAP is beyond the scope of this article; however, some classic associations are included below:
M pneumoniae CAP is associated with the following findings [54] :
-
Headache, fever, malaise, sore throat in young adult with insidious onset of cough
-
Erythema multiforme major (Stevens-Johnson syndrome)
-
Cardiac conduction abnormalities
-
Hemolytic anemia and cold-agglutinin syndrome
-
Neurologic abnormalities, including aseptic meningitis or meningoencephalitis, Guillain-Barre syndrome, transverse myelitis
-
Epidemic outbreaks, such as schools or military barracks
Legionella pneumophila CAP (Legionnaires disease) is associated with the following findings [55] :
-
Gastrointestinal and neurologic symptoms in the setting of pneumonia
-
Positive history of water or travel exposure
-
Relative bradycardia during febrile episode
-
Hyponatremia, hypophosphatemia, elevated creatine phosphokinase (CPK) level, elevated ferritin level, myoglobinuria
-
Leukocytosis with relative lymphopenia
-
Unresponsive to beta-lactam antibiotics
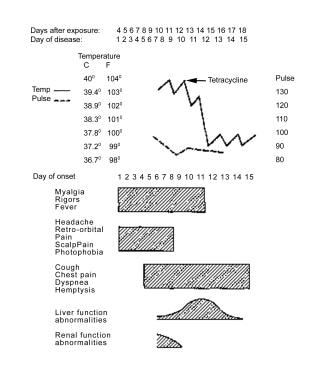 A case of Legionnaires disease from the Philadelphia outbreak, showing characteristics of relative bradycardia and extrapulmonary involvement.
A case of Legionnaires disease from the Philadelphia outbreak, showing characteristics of relative bradycardia and extrapulmonary involvement.
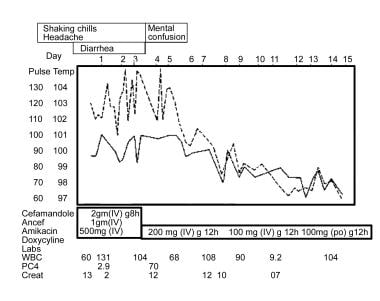 This graph outlines a case of Legionella pneumonia, showing characteristics of bradycardia and extrapulmonary involvement. Also shown is an initial lack of response to beta-lactam antibiotics, followed by effective treatment with doxycycline.
This graph outlines a case of Legionella pneumonia, showing characteristics of bradycardia and extrapulmonary involvement. Also shown is an initial lack of response to beta-lactam antibiotics, followed by effective treatment with doxycycline.
C burnetii CAP (Q fever) is associated with the following factors [56, 57] :
-
Acute infection
-
Severe retrobulbar headache, myalgias, fever, rigors, nonproductive cough
-
Elevated levels of transaminases and thrombocytopenia
-
Maculopapular or purpuric rash
-
Zoonotic exposure (goats, sheep, cattle most common)
Community-acquired pneumonia and shock
With the exception of CAP due to particularly virulent organisms (eg, S aureus, Hantavirus, severe acute respiratory syndrome [SARS-CoV-1 and 2], Legionella), CAP does not typically present with shock in otherwise healthy hosts. Therefore, in addition to considering the possibility of CAP due to a hypervirulent pathogen, patients who present with fever, dyspnea, leukocytosis, pulmonary infiltrates, and shock in the absence of conditions associated with hyposplenism should be evaluated for imitators of pneumonia, such as acute myocardial infarction or acute pulmonary embolism.
Conditions that predispose to severe CAP should be considered in patients presenting with CAP and shock in the absence of one of the aforementioned cardiopulmonary diseases. The following disorders and therapies have been associated with severe CAP:
-
Hyposplenism, splenectomy, or congenital asplenia
-
Corticosteroid therapy
-
HIV/AIDS
-
Chronic alcoholism
-
Amyloidosis
-
Chronic active hepatitis
-
Other causes of immunocompromise: Immunoglobulin A (IgA) deficiency, intestinal lymphangiectasia, myeloproliferative disorders, Waldenström macroglobulinemia, systemic mastocytosis
-
Illnesses requiring immunosuppression: Celiac disease, rheumatoid arthritis, s ystemic lupus erythematosus ( SLE), vasculitis, non-Hodgkin lymphoma
Patient History
Patients with community-acquired pneumonia (CAP) due to typical bacterial CAP pathogens commonly present with fever, dyspnea, and productive cough, often with pleuritic chest pain.
The clinical presentation of atypical CAP is more subacute and associated with extrapulmonary manifestations that may provide a clue to the etiology.
Zoonotic infection
Contact with the appropriate zoonotic vector or its by-product (eg, milk, urine, feces, placenta) is needed to develop a zoonotic CAP. A history of occupational exposure to livestock (eg, farmers, veterinarians) or close contact with a parturient animal should be sought in patients with suspected Q fever. Psittacosis is preceded by recent contact with birds infected with C psittaci. Occupations and avocations associated with increased risk include poultry farming, pet shops, veterinary clinics, and ownership of pet birds (classically of the psittacine, or parrot, family). Hantavirus is transmitted via exposure to wild rodents, specifically to aerosolized rodent urine or feces; thus, queries as to whether a patient presenting with severe CAP works or recreates in a setting conducive to rodent exposure (eg, farms, ranches, forests) is warranted.
Physical Examination
Purulent sputum is characteristic of pneumonia caused by typical bacterial community-acquired pneumonia (CAP) pathogens and is not usually a feature of pneumonia caused by atypical pathogens, with the exception of Legionnaires disease. Blood-tinged sputum may be found in patients with pneumococcal pneumonia, Klebsiella pneumonia, or Legionella pneumonia.
Rales are heard over the involved lobe or segment. Consolidation may be accompanied by an increase in tactile fremitus, bronchial breath sounds, and egophony.
Legionella pneumonia, Q fever, and psittacosis are atypical pneumonias that may present with signs of consolidation. Consolidation is not a typical feature of pneumonia caused by M pneumoniae or C pneumoniae. [47]
Be wary when a patient presents with severe CAP, with or without hypotension or shock. In these patients, be sure to exclude underlying immunocompromise, asplenia, or acute pulmonary or cardiac event or a particularly virulent pathogen such as influenza or S aureus that could explain the severity of the CAP.
Pleural effusion
Pleural effusion, if large enough, may be detectable on physical examination. Patients with a pleural effusion have decreased tactile fremitus, decreased breath sounds on auscultation, and dullness on chest percussion.
Empyema
On physical examination, empyema has the same findings as pleural effusion. Thoracentesis with analysis of pleural fluid for pH, cell counts, and Light's criteria are helpful when differentiating simple pleural effusions, parapneumonic effusions, or empyemas. PCR, in addition to traditional culture methods, may be helpful to determine the bacterial pathogen, if necessary. [58]
Differential Diagnoses of Community-Acquired Pneumonia
Aside from those mentioned above, the differential diagnoses to consider in the diagnosis of community-acquired pneumonia (CAP) include the following:
-
Acute bronchitis
-
Acute exacerbation of chronic bronchitis
-
Aspiration pneumonitis
-
Tracheobronchitis
-
Myocardial infarction
-
CHF and pulmonary edema
-
Pulmonary fibrosis
-
Sarcoidosis
-
SLE pneumonitis
-
Pulmonary drug hypersensitivity reactions (nitrofurantoin, daptomycin)
-
Drug-induced pulmonary disease (bleomycin)
-
Cryptogenic organizing pneumonia
-
Pulmonary embolus or infarction
-
Bronchogenic carcinomas
-
Radiation pneumonitis
-
Granulomatosis with polyangiitis (Wegener granulomatosis)
-
Lymphoma
Sputum Studies and Blood Culture
Send sputum samples from patients with severe community-acquired pneumonia (CAP) for Gram stain and/or culture. Keep in mind that many patients, especially elderly persons, may not be able to produce an adequate suitable sputum sample.
Sputum Gram stain is reliable and diagnostic if performed on a well-collected specimen without many squamous epithelial cells (saliva contamination) and if a predominant organism is present. Gram stain shows few or no predominant organisms in patients with atypical CAP.
In all patients with CAP who meet criteria for hospital admission, obtain blood cultures prior to administering antibiotics because pneumonia due to some bacterial pathogens, such as S pneumoniae and H influenzae, is frequently associated with positive blood cultures. The overall yield from blood cultures is low, as viral and many bacterial pathogens such as M catarrhalis are not often associated with bacteremia. [27]
Studies in HIV-Positive Patients with Community-Acquired Pneumonia
The differential diagnoses of community-acquired pneumonia (CAP) in patients with human immunodeficiency virus (HIV) infection is broader than in HIV-negative patients. The patient’s immunologic status, as reflected by the CD4 count, the clinical course, and the chest radiographic appearance, provides clues to the most likely etiologic organism.
Patients with HIV infection and a normal or slightly decreased CD4 count with focal infiltrates have approximately the same pathogen distribution as otherwise healthy hosts (eg, S pneumoniae is most common) and thus warrant the same diagnostic strategies as the general population. Pneumocystis jiroveci pneumonia (PJP) should be suspected in patients with a CD4 count of less than 200 cells/µL (or CD4% < 14%) presenting with gradually progressive dyspnea, nonfocal infiltrates on chest radiography, nonproductive cough, and hypoxemia. A definitive diagnosis of PJP requires visualization of the PJP cysts (ie, using special stains such as Giemsa or methenamine silver). Bronchoscopy with bronchoalveolar lavage (BAL) is often necessary to obtain an adequate specimen. The utility of other diagnostics such as serum 1,3 B-D glucan or Pneumocystis PCR of the BAL is still being studied. Current limitations include the lack of reliable thresholds for 1,3 B-D glucan testing for the diagnosis of Pneumocystis as well as cost and unclear significance of lower quantitative values with PCR. [59, 60]
In the absence of an alternate etiology such as influenza, patients with HIV infection who are admitted with CAP should be placed on airborne isolation while the possibility of active pulmonary tuberculosis is evaluated (eg, with sputum AFB smears/cultures), factoring in local incidence and potential exposure risk.
Additional uncommon CAP pathogens, such as histoplasmosis, coccidioidomycosis, and cryptococcosis, should also be considered in HIV-positive patients who present with pulmonary infiltrates, with risk stratified according to potential exposure history. Antigen testing (urine antigen for histoplasmosis; serum antigen for cryptococcosis) may be helpful in these cases.
Other Laboratory Tests for Community-Acquired Pneumonia
Several nonspecific laboratory tests are often performed during the workup of community-acquired pneumonia (CAP), particularly if atypical CAP is suspected.
Serum transaminase, serum sodium, serum ferritin, serum phosphorus, and creatine phosphokinase (CPK) levels may provide evidence supporting a particular pathogen, such as Legionella. Lactic acid, white blood cell (WBC) count, blood urea nitrogen, and creatinine may be used in categorizing the severity of illness.
Cold agglutinin titers of 1:32 or greater may support a diagnosis of M pneumoniae, although this test is neither sensitive nor specific and has largely been replaced by newer diagnostic studies, such as respiratory pathogen PCR assay.
Procalcitonin and C-reactive protein levels
C-reactive protein (CRP) and procalcitonin (PCT) are inflammatory biomarkers that have been extensively studied in recent years as possible adjuncts for the diagnosis and management of pneumonia.
CRP is widely available and is relatively inexpensive, although, as a nonspecific acute-phase reactant, it has limited ability to differentiate between causes of inflammation.
PCT is a calcitonin preceptor peptide that is released in response to microbial endotoxins and pro-inflammatory cytokines triggered with bacterial infection (interleukin-1beta, tumor necrosis factor alpha, and interleukin 6), while being down-regulated by interferon gamma, a cytokine released in response to viral infections. [12] Thus, there has been significant interest in whether procalcitonin may be able to help quickly determine a bacterial etiology of infection. PCT has shown promise in diagnosing bacterial versus nonbacterial infection, predicting CAP severity and prognosis, and guiding duration of antibiotic therapy in pneumonia. However, at this point, significant controversy remains regarding the practical utility of procalcitonin in clinical practice. Various factors contribute to the dispute, including limitations of the test itself such as absence of systemic release of PCT in localized infections, insufficient availability, or delay in results of the PCT assay at many treating facilities, conflicting results among studies, and lack of consistent established cutpoints to guide management decisions. Further prospective studies are needed to help clarify these and other issues, such as cost-effectiveness.
Several studies to date have demonstrated PCT levels alone or in combination with CRP may help predict mortality in CAP. [14] The time from symptom onset influences both CRP and PCT levels at the time of CAP diagnosis, [61] which may need to be considered when determining how to apply PCT values to prognostic and/or management decisions.
A 2018 meta-analysis of patient data from 26 randomized controlled trials assessed the safety of procalcitonin-guided antibiotic decisions (relating to either initiating or continuing antibiotics) in patients with acute respiratory infections. [62] Among the 6,708 patients whose data were included, 30-day all-cause mortality rates were significantly lower (adjusted odds ratio [aOR], 0.83) in patients who were randomized to the PCT-guided treatment arm compared to controls, as was duration of antibiotic exposure (5.7 vs 8.1 days; P < 0.001) and frequency of adverse effects due to antibiotic therapy (16% vs 22%; aOR, 0.68). However, there was not a statistically significant difference in rate of treatment failure between the PCT-guided and control groups (aOR, 0.9). This meta-analysis included a heterogeneous group of respiratory tract diagnoses, including acute exacerbations of COPD and bronchitis, in addition to CAP, hospital-acquired pneumonia (HAP), and ventilator-associated pneumonia (VAP). Although CAP represented fewer than half of the cases, no significant difference in all-cause 30-day mortality rate was detected when the analysis was restricted to patients with a diagnosis of CAP (aOR, 0.82). Treatment failure and antibiotic side effects were significantly less common in the PCT-guided group, yet length of ICU stay was longer. Limitations of this meta-analysis included variable adherence to the PCT algorithm and inconsistent PCT thresholds used to help guide antibiotic therapy among the studies, potentially affecting applicability to clinical practice.
A 2020 retrospective study reviewing patients admitted with influenza and abnormal chest radiography noted that while patients with a positive PCT did receive more antibiotics compared to those with a negative PCT, there was no difference in 30-day readmission rates or 90-day mortality between those who received antibiotics and those who did not. The authors suggest the use of a negative procalcitonin for de-escalation of antibiotics could be studied further for the purposes of improved antibiotic stewardship, though this study was also limited by the retrospective methodology and smaller sample size. [63] Other studies suggest that use of PCT for antibiotic de-escalation may be more beneficial in those with an uncertain diagnosis of CAP. [64]
The 2016 IDSA/ATS guidelines on HAP/VAP recommended against the addition of either PCT or CRP to clinical criteria alone when deciding whether to initiate antibiotics, although they did provide a weak recommendation for the use of PCT levels plus clinical criteria to help guide discontinuation of antibiotics in patients with VAP. [65] The 2019 IDSA/ATS guidelines on CAP similarly advise against the routine addition of PCT to clinical judgment alone for the decision to initiate antibiotics. This was primarily based on the wide-ranging sensitivity of the test for bacterial pneumonia, variable thresholds for a positive test between different studies, and lack of use of radiographically confirmed pneumonia in many studies. [7]
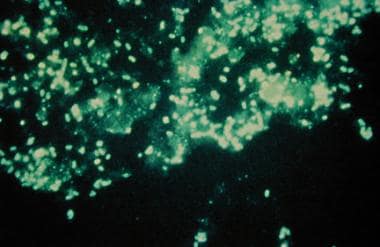
Direct fluorescent antibody testing
Direct fluorescent antibody (DFA) testing of the sputum can be performed to try to assist with the diagnosis of atypical CAP pathogens, including Legionella, P jiroveci, and Chlamydophila; however, the utility of this test is hampered by suboptimal sensitivity and is relatively difficult to perform. A DFA stain showing Legionella infection is seen in the image below.
Serology
Serology has been replaced by more rapid PCR-based assays in many settings; serology was historically useful in identifying fastidious organisms, particularly when dealing with pathogens that have potential epidemiologic implications (ie, epidemic or biohazard situations), such as B pertussis, M pneumoniae, L pneumophila, or C burnetii. Serologic diagnosis is based on a 4-fold or greater increase in titers between acute- and convalescent-phase serum specimens so is generally not useful in the acute-care setting. A significantly elevated IgM titer (typically present in the acute phase of infection) may help support a diagnosis, and some experts have suggested that combining IgM and nucleic acid amplification testing may be the optimal method for diagnosing M pneumoniae infection. [66]
Urinary antigen test
Pneumococcal urinary antigen testing (UAT) is a non–culture-based test for diagnosing pneumococcal infection that has a reported sensitivity of 50% to 80% and specificity of more than 90%. [67] Thus, a negative result does not rule out pneumococcal pneumonia. The UAT results remain positive after antibiotics have been started so may be particularly useful when cultures were not obtained prior to initiation of antibiotic therapy. Recent IDSA guidelines recommend use of pneumococcal urinary antigen testing in adults with severe CAP. [7]
In 2018, Wunderink et al reported that serotype-specific urinary antigen detection assays may further improve detection of pneumococcal pneumonia in hospitalized adults with CAP. [68] Availability of pneumococcal UAT is the main perceived barrier to its routine use. [67]
Urinary antigen testing is considered the first-line diagnostic test for L pneumophila. The sensitivity of the test ranges from 55% to 99%, with improved sensitivity paralleling disease severity. [55] The specificity is high (95%). The urinary antigen test is applicable only for L pneumophila serogroup type I, which accounts for approximately 85% of Legionella infections in the United States and Europe. In other regions of the world, including Australia, Thailand, and New Zealand, 25% of cases have been attributed to another species, Legionella longbeachae. [69] The UAT results remain positive for Legionella for long periods, but may be negative within the first 48 hours of infection. Using a combination of Legionella UAT and PCR of respiratory secretions, which is often able to detect all species of Legionella, may increase the ability to diagnose Legionella pneumonia. [70] The 2019 IDSA/ATS CAP guidelines recommend obtaining a urine Legionella antigen test in patients with severe CAP and when epidemiologic factors support a potential Legionella diagnosis. [7]
Polymerase chain reaction
Polymerase chain reaction (PCR) has emerged as an important diagnostic tool for determining the etiology of CAP, particularly with regard to respiratory viruses and fastidious organisms, including Legionella, Mycoplasma, and Chlamydophila. [2] PCR is a very sensitive and specific method for isolating these pathogens. The source of specimen may affect the diagnostic yield of PCR assays; for instance, the detection rate of many pathogens, including Legionella and M pneumoniae, is higher with sputum samples than with nasopharyngeal aspirates. [70] However, nasopharyngeal samples remain useful, as many patients are unable to provide a quality sputum sample. The increasing commercial availability of various PCR assays (including multiplex) has allowed for increased implementation in the clinical setting. Multipathogen approaches are less expensive and more time consuming than testing each organism individually, although they may be complex to perform. Rapid molecular tests for respiratory viruses are also increasingly being developed with the goal of further shortening the time to diagnosis. [71]
Interpreting the significance of PCR detection of an organism known to colonize the upper airway or those that may be associated with protracted shedding, such as rhinovirus, remains a challenge. Quantitative PCR methods have shown some promise in improving interpretability of such findings. [72] PCT has also been used in conjunction with a lower respiratory multiplex PCR panel to distinguish bacterial infection from colonizers. [73]
A 2016 study in the United Kingdom assessed the use of comprehensive molecular testing of a single lower respiratory tract specimen to detect a pathogen in CAP. [74] The study compared (1) a combination of real-time multiplex PCR assays targeting 26 different pathogens, including bacteria and viruses, with (2) culture-based diagnostic testing of the same specimens. Quantification of bacterial load was performed for eight common bacteria, including S pneumoniae, H influenzae, and M catarrhalis. A pathogen was detected in 87% of patients with CAP using the multiplex assay, whereas culture achieved a diagnosis in only 39% of cases. Viruses were detected in 30% of cases. H influenzae and S pneumoniae were the most commonly detected bacteria. Pathogen detection was not significantly decreased when a quantitative threshold for detection of ≥105 CFU/mL for all bacterial loads was applied, although the mean bacterial load was lower in patients who had received prior antibiotics than in those who had not. Interestingly, of the 268 patients who received antibiotics prior to testing, 77.6% had a positive bacterial PCR finding, but only 32% were culture-positive. The authors concluded that comprehensive molecular testing on a lower respiratory tract specimen has the potential to positively affect targeted antibiotic therapy in most patients with CAP.
The 2020 IDSA guidelines on molecular testing for respiratory tract infections suggest that lower respiratory tract bacterial NAAT may be most beneficial in patients with intermediate pretest probability and disease severity, as results would be more likely to influence targeting antibiotics appropriately in the case of a positive test versus avoidance of unnecessary empiric antibiotics with a negative test. However, more extensive study is needed on the topic, including benefits of quantitative results to potentially distinguish between colonization and true infection, as well as cost-effectiveness of testing. [75] Alternatively, the 2021 ATS guidelines on non-influenza viral NAAT in patients with CAP advise performing viral multiplex testing only in patients with severe CAP or who are immunocompromised. This is attributed to limited evidence that a test result, whether positive or negative, impacts subsequent antibiotic therapy outside of these high-risk populations. [76]
Chest Radiography and CT Scanning
Chest radiography
Obtain chest radiographs in all patients with suspected community-acquired pneumonia (CAP) to exclude conditions that mimic CAP and to confirm the presence of an infiltrate compatible with the presentation of CAP. [8, 9]
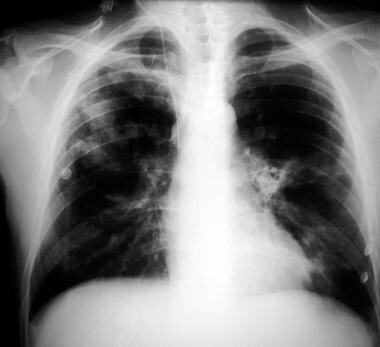
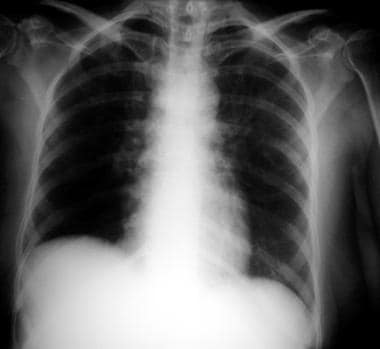 Chest radiograph in a patient with HIV infection, bilateral perihilar infiltrates, and Pneumocystis jiroveci pneumonia.
Chest radiograph in a patient with HIV infection, bilateral perihilar infiltrates, and Pneumocystis jiroveci pneumonia.
Chest radiography findings may be negative in patients who present very early with CAP. In these patients, repeat chest radiography within 24 hours is recommended.
Chest radiography may assist with the differentiation of viral pneumonias from nonviral pneumonias but are not specific enough alone to confirm the diagnosis. Viral pneumonias tend to display few or no infiltrates on chest radiography, but, when infiltrates are present, they are almost always bilateral, perihilar, symmetric, and interstitial. The appearance of infiltrates in viral pneumonia may be mistaken for pulmonary edema, or vice versa.
Bacterial pneumonias have a predominantly focal segmental or lobar distribution, with or without pleural effusions. Atypical bacterial pathogens have variable radiographic findings, ranging from focal segmental to bilateral interstitial disease. P jiroveci pneumonia (PJP) typically manifests as bilateral patchy interstitial infiltrates. Of note, radiographic findings alone are not reliable for differentiating specific etiologies of CAP.
Chest radiographic findings should be negative in patients with asthma or exacerbation of chronic bronchitis who do not have CAP.
The infiltrates observed with CHF appear as increased interstitial markings and show vascular redistribution to the upper lobes. Patients with preexisting heart failure usually have cardiomegaly.
Rapid cavitation is not a typical feature of CAP.
Methicillin-resistant S aureus (CA-MRSA) CAP presents as a fulminant CAP with rapid cavitation and necrotizing pneumonia. MRSA CAP sometimes occurs after influenza but may also develop independent of influenza, even in young healthy hosts.
Serial chest radiography can be used to observe the progression of severe CAP if the patient has not improved within 5 to 7 days. [7] Chest radiographic findings worsen rapidly in severe typical bacterial CAP and require a significant period to improve. It is important to note that clinical resolution occurs long before radiologic resolution.
CT scanning
Obtain a computed tomography (CT) scan of the chest if pneumonia is clinically suspected despite persistently negative chest radiographic findings to examine for viral, atypical, or mycobacterial pneumonia, particularly in immunocompromised hosts with a broad differential diagnosis that would benefit from further narrowing. When an underlying bronchogenic carcinoma is suspected or if any abnormalities are not consistent with the diagnosis of pneumonia, CT is helpful to examine for structural lung disease or malignancy.
Fine-Needle Aspiration and Bronchoscopy With Bronchoalveolar Lavage
Diagnostic bronchoscopy with bronchoalveolar lavage (BAL) may be useful in patients with community-acquired pneumonia (CAP) when Pneumocystis, mycobacteria, or fungal pathogens are likely. Diagnostic bronchoscopy with BAL with or without biopsy may also be useful in unresolving CAP that does not respond to appropriate therapy.
Transthoracic fine-needle aspiration (FNA) of the infiltrate can be performed and is most useful in determining the cause of nodules or noninfectious infiltrates that are not responding to antibiotic treatment.
Histologic Findings
Lung sections with typical bacterial pneumonias show the progression from red hepatization to white hepatization during the resolution process. The lung is repaired or scarred after bacterial pneumonia is complete and the infectious process resolves.
Pharmacologic Therapy
Most experts feel that antimicrobial coverage should be divided against typical and atypical CAP pathogens with consideration for risk of viral infection such as influenza, based on the season. [77]
A 2017 review presented a CAP bundle that is in line with international guidelines and IDSA/ATS guidelines. The 2019 updated IDSA/ATS guidelines also support these components. The bundle includes the following [5, 7] :
-
Use of PSI and clinical criteria to the determine CAP severity and appropriate level of care
-
Rapid empiric appropriate antibiotics started promptly
-
Rapid fluid and electrolyte resuscitation, thromboembolic prophylaxis, and management of hypoxia
-
Early ambulation
-
Assessment of cardiovascular risk with appropriate initiation of aspirin or other prophylaxis
Adequate therapy for CAP includes coverage for S pneumoniae and atypical bacterial pathogens. Treatment options for CAP in outpatients with no comorbidities and no risk factors for drug-resistant S pneumoniae include the following [7] :
-
Amoxicillin 1 gram PO three times a day OR
-
A macrolide (azithromycin 500 mg once and then 250 mg daily or clarithromycin 500 mg twice daily) OR
-
Doxycycline 100 mg twice daily
During influenza season, it is also reasonable to initiate oseltamivir, zanamivir, peramivir, or baloxavir therapy in outpatients who present with a flu-like illness and pneumonia. Macrolides should be used only in areas where pneumococcal resistance is lower than 25%.
Treatment options for CAP in patients with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; asplenia; immunosuppression; prior antibiotics within 90 days; or other risk factors for drug-resistant infection include the following:
-
Beta-lactam (amoxicillin/clavulanate 2 g/125 mg twice daily or 500 mg/125 mg three times daily or 875 mg/125 mg twice daily, cefpodoxime 200 mg twice daily, or cefuroxime 500 mg twice daily) AND a macrolide or doxycycline OR
-
Respiratory fluoroquinolones (moxifloxacin 400 mg daily, levofloxacin 750 mg daily)
For hospitalized patients, therapy consists of the following:
-
Beta-lactams (ampicillin/sulbactam 1.5-3 g every 6 hours or ceftriaxone 1-2 g daily or cefotaxime 1-2 g every 8 hours or ceftaroline 600 mg every 12 hours) AND a macrolide OR
-
Respiratory fluoroquinolone OR
-
If macrolides and fluoroquinolones are contraindicated: beta-lactam as above AND doxycycline
Therapy in ICU patients includes the following:
-
Beta-lactam (ceftriaxone, cefotaxime, or ampicillin/sulbactam) AND a macrolide or respiratory fluoroquinolone OR
-
In patients with penicillin allergy: a respiratory fluoroquinolone AND aztreonam
Risk factors for Pseudomonas pneumonia include structural lung disease, COPD, and bronchiectasis. [4] If Pseudomonas is suspected, therapy is as follows:
-
Anti-pneumococcal and anti-pseudomonal beta-lactam ( piperacillin/tazobactam 4.5 g every 6 hours or cefepime 2 g every 8 hours or ceftazidime 2 g every 8 hours or meropenem 1 g every 8 hours or imipenem 500 mg every 6 hours.
-
In patients with severe penicillin allergy, aztreonam 2 g every 8 hours may be used instead of the beta-lactam in the regimen listed above. It is worth noting that many reported penicillin allergies are not true allergies. Owing to the limited spectrum of aztreonam and the relatively low likelihood of penicillin allergy cross-reacting with cephalosporins (2%), cefepime is a reasonable choice after considering the balance of benefit and risk. [18]
If MRSA is suspected, vancomycin 15 mg/kg every 12 hours adjusted based on levels or linezolid 600 mg every 12 hours should be added. Risk factors for MRSA include hemoptysis, recent influenza, neutropenia, hemodialysis, and congestive heart failure. [4] During influenza season, it is also reasonable to start oseltamivir, zanamivir, peramivir, or baloxavir therapy to treat influenza in patients with MRSA CAP, as well as in patients who present with a flulike illness and pneumonia, as influenza may have preceded the MRSA infection. However, oseltamivir remains the preferred agent in inpatients with suspected or confirmed influenza, as there is limited data to support the alternative antivirals in severe or complicated disease. [19]
Other antibiotics approved by the FDA for CAP
Omadacycline is a tetracycline designed to overcome tetracycline resistance, and it was shown to be noninferior to moxifloxacin. [78, 79]
Lefamulin, a pleuromutilin antibiotic, was found to be noninferior to moxifloxacin in a 2019 phase 3 trial. [80] Lefamulin is indicated for the treatment of bacterial CAP due to S pneumoniae, S aureus (methicillin-susceptible isolates), H influenzae, Legionella pneumophila, M pneumoniae, or C pneumoniae in adults. It is administered twice daily as either an intravenous infusion or an oral tablet.
Solithromycin, a new macrolide, was compared with moxifloxacin as an IV-to-PO option and was found to be noninferior. [81]
Ceftaroline has been shown noninferior and possibly superior to ceftriaxone for CAP in randomized, controlled, double-blind, international trials based in the United States and Asia. [82, 83]
Delafloxacin, a novel fluoroquinolone, has the advantage of activity against both MRSA and Pseudomonas, and gained approval in October 2019 for the treatment of bacterial CAP in adults. Approval was based on a phase 3 randomized, double-blind study (n = 859) that compared delafloxacin with moxifloxacin. Results showed that IV-to-oral delafloxacin was noninferior at 96 hours compared with moxifloxacin. [85, 86]
Ceftobiprole medocaril sodium is an extended-spectrum cephalosporin with activity against clinically important gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). It is indicated for S aureus bacteremia (SAB) in adults, including right-sided infective endocarditis, caused by methicillin-susceptible and methicillin-resistant isolates.
Treatment Duration and other Pharmacologic Considerations
Mild to moderately ill patients with CAP may be treated entirely via the oral route, on either an inpatient or outpatient basis. Patients who are severely ill or unable to tolerate or absorb oral medications require some duration of intravenous therapy before switching to an oral antibiotic. [26]
If the patient is switched to an oral regimen and is doing well, earlier discharge with completion of antibiotics at home is possible. Optimal intravenous-to-oral switch therapy consists of a single agent that has an appropriate spectrum, has excellent bioavailability, is well tolerated, has a low resistance potential, and is relatively inexpensive.
The duration of therapy for uncomplicated CAP is usually 5 days. [20, 27] The duration of therapy for CAP due to suspected or proven Pseudomonas or MRSA is typically 7 days. Patients should be afebrile for 48 to 72 hours and have no signs of instability before antibiotic therapy is stopped. The duration of therapy may need to be increased if the initial empiric therapy has no activity against the specific pathogen or if the pneumonia is complicated by extrapulmonary infection. [27] The use of systemic corticosteroids in patients with CAP is not supported routinely but may be useful for managing certain comorbidities or as part of the sepsis bundle. Studies have shown that corticosteroids may reduce the length of time until clinical stability, decrease hospital length of stay, lessen the need for mechanical ventilation, and lower the incidence of adult respiratory distress syndrome. [87, 88] These potential benefits should be weighed against the risk of worsening influenza pneumonia or causing hyperglycemia. [5] Furthermore, there is insufficient data or agreement on the dose or duration of the steroid therapy.
Appropriate spectrum
In otherwise healthy hosts with CAP, therapy does not need to cover S aureus, Klebsiella species, or P aeruginosa. Coverage should include typical (S pneumoniae, H influenzae, M catarrhalis) and atypical (Legionella and Mycoplasma species, C pneumoniae) pathogens. S aureus coverage should be included in patients with influenza who have focal infiltrates or patients who have risk factors for MRSA pneumonia such as prior hospital admission of 2 or more days in the preceding 90 days, antibiotics in the preceding 90 days, residence in a long-term care facility, septic shock at the time of CAP, immunosuppression, enteral tube feedings, nonambulatory status, gastric acid suppression, chronic hemodialysis, congestive heart failure, and history of MRSA colonization. [5, 65]
Most antibiotics used to treat community-acquired aspiration pneumonia (eg, beta-lactam/beta-lactamase inhibitor) are highly effective against oral anaerobes. For aerobic lung abscesses, clindamycin or moxifloxacin is preferable. [89, 90, 91, 92]
Monotherapy
Preferred monotherapy for mild or outpatient CAP includes a macrolide, amoxicillin, or doxycycline. For patients with an increased risk for resistance or with numerous comorbidities, monotherapy options include respiratory quinolone. Prior to the use of a fluoroquinolone, an assessment of contraindications and risk of adverse effects should be performed.
Penicillin resistance
Most penicillin-resistant S pneumoniae infections may be treated with beta-lactams. Alternately, doxycycline or respiratory quinolones may be used.
High-level penicillin-resistant S pneumoniae (MIC 6 µg/mL) strains are a rare cause of CAP; fortunately, they remain susceptible to ceftriaxone.
Severity
The severity of CAP may be estimated with scoring systems. The Pneumonia Severity Index (PSI) is preferred over the CURB-65 (confusion, uremia, respiratory rate, low blood pressure, age >65 years) for determining outpatient verses inpatient treatment. Patients with PSI class IV-V may need hospitalization or more intensive in-home services. Severe pneumonia is defined as having 1 major criteria or 3 minor criteria as follows:
Major criteria:
-
Septic shock requiring vasopressors
-
Respiratory failure requiring mechanical ventilation
Minor criteria:
-
Respiratory rate of 30 or more breaths per minute
-
PaO 2/FIO 2 ratio of 250 or less
-
Multilobar infiltrates
-
Confusion
-
Uremia
-
Leukopenia (WBC < 4000 cells/µl)
-
Thrombocytopenia (platelet count < 100,000/µl)
-
Hypothermia
-
Hypotension
These are the primary criteria to use to determine if a patient requires ICU admission. CURB-65 was designed to predict mortality risk; CRB-65 is similar but is used when certain laboratory tests are unavailable. Some scoring systems (eg, CURXO and SMART-COP) focus on the severity of CAP and likelihood of requiring ventilatory or circulatory support, whereas others (eg, PSI score) help determine whether CAP requires hospitalization.
Comorbid conditions
Comorbid conditions affect a patient’s risk of contracting CAP caused by MRSA, Pseudomonas, or other antibiotic-resistant pathogens. Risks for antibiotic-resistant pathogens include hospital admission within the preceding 90 days, antibiotics in the preceding 90 days, septic shock at the time of CAP, immunosuppression, enteral tube feedings, non-ambulatory status, or gastric acid suppression. MRSA risk factors include all of the prior plus hemodialysis, CHF, and a history of MRSA colonization. [5, 65]
There is conflicting evidence as to the safety of using PPIs and H2 blockers. [93, 94, 95, 96] Some studies have concluded that PPI use increases the risk for pneumonia or even the risk of multidrug-resistant pathogens causing pneumonia. Other, more recent, studies find such links may have resulted from confounding factors. [95, 97]
Negative prognostic factors in community-acquired pneumonia (CAP) include preexisting lung disease, underlying cardiac disease, poor splenic function, advanced age, multilobar involvement, past infection with tuberculosis, and delayed initiation of appropriate antimicrobial therapy. [17, 98]
Overall, comorbidities represent important prognostic factors and contribute to the severity index. [98]
Outpatient Care in Community-Acquired Pneumonia
Patients with mild community-acquired pneumonia (CAP) who are being treated on an outpatient basis should be monitored to ensure compliance with their medications and to ensure clinical improvement. After 1 week, a repeat visit is advisable. If the patient is improving and parapneumonic complications are not evident, posttherapy chest radiography is unnecessary. [99, 100, 101]
The diet in patients with CAP is as tolerated. Guide activity with common sense.
Vaccination
Pneumococcal vaccines have been shown to have some efficacy in preventing vaccine-strain pneumococcal pneumonia, bacteremia, and invasive disease. They have not been shown to prevent community-acquired pneumonia (CAP) of all kinds. [102, 103] Annual influenza vaccination has been shown to decrease pneumonia diagnoses, hospitalizations, and cardiac events in certain populations. [104, 105, 106, 107]
Annual influenza vaccination is recommended in all persons older than 6 months. There are several options for vaccination, including standard-dose trivalent inactivated vaccine, high-dose inactivated trivalent vaccine, three different formulations of quadrivalent inactivated vaccine (egg-based, cell culture–based, and recombinant-derived), and a live attenuated influenza vaccine. Per current CDC recommendations, people with a history of egg allergy of any severity may receive any licensed, recommended, and age-appropriate influenza vaccine. [108] Individuals with a history of severe egg allergy should receive the vaccine under the supervision of a physician experienced in the management of allergic conditions. People with a personal history of severe allergy to the flu vaccine should not receive it, and persons with history of Guillain-Barré syndrome should consult with their physician prior to being vaccinated. [109]
Four pneumococcal vaccines are approved in the United States:
-
13-valent polysaccharide conjugate vaccine (PCV13; Prevnar 13) is approved for children aged 6 weeks to 17 years and adults aged 50 years or older.
-
15-valent conjugate vaccine (PCV15; Vaxneuvance) is approved for adults 18 years and older.
-
20-valent conjugate vaccine (PCV20; Prevnar 20) is approved for adults 18 years and older.
-
23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax 23) is approved for adults aged 65 years or older and persons aged 2 years or older who are at an increased risk for pneumococcal disease.
The Advisory Committee on Immunization Practices (ACIP) recommends:
-
Healthy adults 65 years or older without known history of receiving PCV should receive 1 dose of PCV20 alone or 1 dose of PCV15 followed by PPSV23 at least one year later.
-
Adults 19 years and older with immunocompromising conditions (such as HIV, cancer, renal disease), functional or anatomic asplenia, cerebrospinal fluid leaks, or cochlear implants without known history of receiving PCV should receive 1 dose of PCV20 alone or 1 dose of PCV15 followed by a dose of PPSV23 at least one year later. Immunocompromised patients, those with cochlear implants or CSF leak may benefit from a shortened duration to 8 weeks.
- Vaccinations do not need to be repeated if given prior to age 65.
-
In patients with prior PPSV23 only, 1 dose of PCV20 or PCV15 may be given at least one year after the most recent PPSV23 dose.
-
There are no current recommendations for administration of PCV15 or PCV 20 in patients with prior PCV13 alone or PCV13 and PPSV23.
Coadministration of PCV15, PCV20, and PPSV23 with the quadrivalent influenza vaccine is safe, though there is currently insufficient data for a recommendation for coadministration with non-influenza vaccines. [110]
Non-leukopenic immunocompromised hosts, such as those with rheumatoid arthritis, SLE, or alcoholism, may not develop an antibody response to the pneumococcal vaccine and may therefore remain susceptible to pneumococcal pneumonia. The same is true concerning the use of the Haemophilus vaccine.
Infectious Diseases Society of America/American Thoracic Society CAP Guidelines
The Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) issued updated clinical practice guidelines for community-acquired pneumonia (CAP) in October 2019. [7]
Diagnosis
Gram stain and sputum culture
Routine sputum culture and Gram stain are not recommended in adult outpatients with community-acquired pneumonia (CAP).
In hospitalized patients, pretreatment Gram stain and culture of respiratory secretions are recommended in adults with CAP that is considered severe (particularly in intubated patients) or who meet one of the following conditions:
-
Is currently receiving empiric treatment for methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa
-
Previously had MRSA or P aeruginosa infection, especially of the respiratory tract
-
Was hospitalized within the preceding 90 days and received parenteral antibiotics for any reason
Blood culture
Blood cultures are not recommended in adult outpatients with CAP.
Routine blood cultures are not recommended in hospitalized adults with CAP.
Pretreatment blood cultures are recommended in hospitalized adults with CAP that is classified as severe or who meet one of the following conditions:
-
Is currently receiving empiric treatment for MRSA or P aeruginosa
-
Previously had MRSA or P aeruginosa infection, especially of the respiratory tract
-
Was hospitalized within the preceding 90 days and received parenteral antibiotics for any reason
Legionella and pneumococcal urinary antigen testing
Routine urine testing for pneumococcal antigen is not recommended in adults with CAP unless the CAP is severe.
Routine urine testing for Legionella antigen is not recommended in adults with CAP unless the CAP is severe or as indicated based on predisposing epidemiological factors (eg, Legionella outbreak or recent travel).
Legionella testing should consist of urinary antigen assessment and collection of lower respiratory tract secretions for culture on selective media or nucleic acid amplification.
Influenza testing
If influenza is circulating in the community, influenza testing in adults with CAP is recommended with a rapid influenza molecular assay, which is preferred over a rapid influenza diagnostic test.
Procalcitonin testing
Empiric antibiotic therapy is recommended in adults with clinically suspected and radiographically confirmed CAP, regardless of the patient’s initial serum procalcitonin level.
Treatment
Decision for hospitalization
The decision for hospitalization in adults with CAP should be based on clinical judgement plus a validated clinical prediction rule for prognosis. The Pneumonia Severity Index (PSI) is preferred over the CURB-65.
ICU admission
Direct ICU admission is recommended for patients with CAP who have hypotension requiring vasopressors or respiratory failure requiring mechanical ventilation.
In patients who do not require vasopressors or a mechanical ventilator, the IDSA/ATS 2007 minor severity criteria, along with clinical judgment, are suggested for deciding whether to escalate treatment intensity.
Outpatient antibiotic regimens
The following antibiotics are recommended in adult patients with CAP who are otherwise healthy:
-
Amoxicillin 1 g three times daily OR
-
Doxycycline 100 mg twice daily OR
-
In areas with pneumococcal resistance to macrolides < 25%: a macrolide (azithromycin 500 mg on day one and then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended-release 1,000 mg daily)
In outpatient adults with CAP who have comorbidities, the following antibiotic regimens are recommended:
-
Combination therapy:
- Amoxicillin/clavulanate 500 mg/125 mg 3 times daily or amoxicillin/clavulanate 875 mg/125 mg twice daily or 2,000 mg/125 mg twice daily or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily) AND
- A macrolide (azithromycin 500 mg on day one then 250 mg daily or clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]) or doxycycline 100 mg twice daily OR
-
Monotherapy:
- Respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily)
Inpatient antibiotic regimens
The following empiric treatment regimens are recommended in inpatient adults with nonsevere CAP who do not have risk factors for MRSA or P aeruginosa:
-
Combination therapy:
- A beta-lactam (ampicillin plus sulbactam 1.5-3 g every 6 hours or cefotaxime 1-2 g every 8 hours or ceftriaxone 1-2 g daily or ceftaroline 600 mg every 12 hours) AND
- A macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily) OR
-
Monotherapy:
- A respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily)
-
If macrolides and fluoroquinolones are contraindicated:
- A beta-lactam as above AND doxycycline
The following regimens are recommended among inpatient adults with severe CAP without risk factors for MRSA or P aeruginosa (agents and doses as above):
-
A beta-lactam plus a macrolide OR
-
A beta-lactam plus a respiratory fluoroquinolone
Anaerobic coverage for suspected aspiration pneumonia
Routine addition of anaerobic coverage for suspected aspiration pneumonia is not recommended except when lung abscess or empyema is suspected.
Extended-spectrum antibiotic therapy for MRSA or P aeruginosa
For guiding selection of extended-spectrum antibiotic coverage in adults with CAP, the prior categorization of healthcare-associated pneumonia (HCAP) should be abandoned.
Empiric coverage for MRSA or P aeruginosa is recommended in adults with CAP only in the presence of locally validated risk factors. Empiric treatment options for MRSA include vancomycin (15 mg/kg every 12 hours) or linezolid (600 mg every 12 hours). Empiric treatment options for P aeruginosa include piperacillin-tazobactam (4.5 g every 6 hours), cefepime (2 g every 8 hours), ceftazidime (2 g every 8 hours), aztreonam (2 g every 8 hours), meropenem (1 g every 8 hours), or imipenem (500 mg every 6 hours).
If empiric coverage for MRSA or P aeruginosa is being administered to adults with CAP based on published risk factors without local etiological data, empiric coverage should be continued while culture data are obtained.
Corticosteroid therapy
Routine corticosteroid treatment is not recommended in adults with CAP (regardless of severity) or severe influenza pneumonia.
The ATS/IDSA CAP guidelines endorse the Surviving Sepsis Campaign recommendations on using corticosteroids in patients with CAP who have refractory septic shock.
Anti-influenza therapy
Anti-influenza treatment (eg, oseltamivir) should be prescribed to all adults with CAP who test positive for influenza, regardless of hospitalization status.
Antibacterial therapy in patients with influenza
Standard antibacterial treatment should be initially prescribed to adults with clinical and radiographic evidence of CAP who test positive for influenza.
Treatment duration
The duration of antibiotic therapy should be guided by a validated measure of clinical stability. Antibiotics should be continued until stability is achieved, for a total antibiotic duration of at least 5 days.
Follow-up chest imaging
Routine follow-up chest imaging is not recommended in adults with CAP whose symptoms have resolved within 5 to 7 days.
Patient Instructions
Remind patients with community-acquired pneumonia (CAP) to comply with prescribed antimicrobials even after they experience clinical improvement. Avoid dampening the cough reflex, and, except in patients with heart failure, encourage hydration to help facilitate clearance of secretions.
Acknowledgments
The views expressed are those of the authors and do not reflect the official policy of the Department of Veterans Affairs, the Department of the Army, Department of the Navy, the Department of the Air Force, the Department of Defense or the US Government and the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF). Mention of trade names, commercial products, or organizations does not imply endorsement by the US Government.
Questions & Answers
Overview
What is community-acquired pneumonia (CAP)?
What causes community-acquired pneumonia (CAP)?
How is community-acquired pneumonia (CAP) transmitted?
Which individuals are at increased risk for severe community-acquired pneumonia (CAP)?
What are the possible complications of community-acquired pneumonia (CAP)?
What is the relationship between community-acquired pneumonia (CAP) and cardiac complications?
What is community-acquired pneumonia (CAP)?
What is typical community-acquired pneumonia (CAP) and what is its clinical presentation?
What is the role of doripenem in the treatment of community-acquired pneumonia (CAP)?
Which is the most common pathogen causing community-acquired pneumonia (CAP)?
What are the extrapulmonary signs and symptoms of atypical community-acquired pneumonia (CAP)?
How is the etiologic agent for atypical community-acquired pneumonia (CAP) identified?
Which diagnostic studies are performed for community-acquired pneumonia (CAP)?
Which tests may be useful in the diagnosis of community-acquired pneumonia (CAP)?
Which scoring systems are used to assess the severity of community-acquired pneumonia (CAP)?
What are the outpatient treatment options for community-acquired pneumonia (CAP)?
How is community-acquired pneumonia (CAP) treated in patients with comorbidities?
Which therapies are used for inpatient treatment of community-acquired pneumonia (CAP)?
Which therapy is used in the ICU for patients with community-acquired pneumonia (CAP)?
When should therapy be initiated in patients with community-acquired pneumonia (CAP)?
What is the etiology of community-acquired pneumonia (CAP)?
Which bacterial pathogens commonly cause community-acquired pneumonia (CAP)?
What is the most common bacterial pathogen causing community-acquired pneumonia (CAP)?
What is the role of S aureus in the etiology of community-acquired pneumonia (CAP)?
Which atypical bacterial pathogens cause community-acquired pneumonia (CAP)?
Which zoonotic atypical pathogens cause community-acquired pneumonia (CAP)?
Which nonzoonotic atypical pathogens cause community-acquired pneumonia (CAP)?
How common is community-acquired pneumonia (CAP)?
Does the incidence of community-acquired pneumonia (CAP) increase with age?
Which extrapulmonary findings suggest atypical community-acquired pneumonia (CAP)?
Which extrapulmonary findings suggest C burnetii community-acquired pneumonia (CAP) (Q fever)?
What pathogens should be considered when shock is present in community-acquired pneumonia (CAP)?
Which conditions are associated with severe community-acquired pneumonia (CAP)?
What is the clinical presentation of atypical community-acquired pneumonia (CAP)?
Which patient history suggests zoonotic community-acquired pneumonia (CAP)?
How is a pleural effusion detected in community-acquired pneumonia (CAP)?
Which physical findings suggest community-acquired pneumonia (CAP)?
Which atypical community-acquired pneumonia (CAP) may present with signs of consolidation?
How is empyema detected in community-acquired pneumonia (CAP)?
What is the role of sputum studies in the evaluation of community-acquired pneumonia (CAP)?
What is the role of blood cultures in the evaluation of community-acquired pneumonia (CAP)?
What is the role of lab testing in the evaluation of community-acquired pneumonia (CAP)?
What is the role of serology in the evaluation for community-acquired pneumonia (CAP)?
What is the role of PCR assays in the evaluation for community-acquired pneumonia (CAP)?
Are PCR assays effective in detecting pathogens associated with community-acquired pneumonia (CAP)?
What is the role of chest radiography in the evaluation of community-acquired pneumonia (CAP)?
Which radiographic findings suggest viral community-acquired pneumonia (CAP)?
Which radiographic findings suggest bacterial community-acquired pneumonia (CAP)?
What is the role of CT scanning in the evaluation for community-acquired pneumonia (CAP)?
Which histologic findings suggest community-acquired pneumonia (CAP)?
What guidelines have been released for the treatment of community-acquired pneumonia (CAP)?
What are the outpatient treatment options for community-acquired pneumonia (CAP)?
What are treatment options for community-acquired pneumonia (CAP) in patients with comorbidities?
When is treatment with a nonmacrolide indicated for community-acquired pneumonia (CAP)?
How is community-acquired pneumonia (CAP) treated in hospitalized patients?
How is community-acquired pneumonia (CAP) treated in ICU patients?
How is Pseudomonas community-acquired pneumonia (CAP) treated?
When should hospitalized patients with community-acquired pneumonia (CAP) be discharged?
What is the duration of therapy for uncomplicated community-acquired pneumonia (CAP)?
What is the role of systemic corticosteroids in the treatment of community-acquired pneumonia (CAP)?
How is the severity of community-acquired pneumonia (CAP) estimated?
What is the appropriate spectrum of antibiotic therapy for community-acquired pneumonia (CAP)?
Which antibiotics are used to treat community-acquired aspiration pneumonia?
What is the preferred monotherapy for community-acquired pneumonia (CAP)?
How are penicillin-resistant S pneumoniae infections treated in community-acquired pneumonia (CAP)?
What is the role of proton-pump inhibitors (PPIs) in the risk of community-acquired pneumonia (CAP)?
How are patients receiving outpatient treatment for community-acquired pneumonia (CAP) monitored?
Who should receive an annual influenza vaccination?
Which vaccines are approved in the US for the prevention of community-acquired pneumonia (CAP)?
What are the recommendations for pneumococcal vaccination in adults?
Which patients may not develop an antibody response to the pneumococcal vaccine?
What instructions should be given to patients being treated for community-acquired pneumonia (CAP)?
What are the negative prognostic factors of community-acquired pneumonia (CAP)?
When is hospitalization indicated for patients with community-acquired pneumonia (CAP)?
-
Gram stain showing Streptococcus pneumoniae.
-
Gram stain showing Haemophilus influenzae.
-
Gram stain showing Moraxella catarrhalis.
-
Clinical diagnostic approach in community-acquired pneumonias.
-
Sputum direct fluorescent antibody stain showing Legionella infection.
-
A case of Legionnaires disease from the Philadelphia outbreak, showing characteristics of relative bradycardia and extrapulmonary involvement.
-
This graph outlines a case of Legionella pneumonia, showing characteristics of bradycardia and extrapulmonary involvement. Also shown is an initial lack of response to beta-lactam antibiotics, followed by effective treatment with doxycycline.
-
Chest radiograph in a patient with HIV infection, bilateral perihilar infiltrates, and Pneumocystis jiroveci pneumonia.
-
Chest radiograph in a patient with HIV infection and focal infiltrates due to tuberculosis.
Tables
What would you like to print?
- Practice Essentials
- Overview
- Etiology of Community-Acquired Pneumonia
- Epidemiology of Community-Acquired Pneumonia
- Extrapulmonary Findings in Atypical Community-Acquired Pneumonia
- Patient History
- Physical Examination
- Differential Diagnoses of Community-Acquired Pneumonia
- Sputum Studies and Blood Culture
- Studies in HIV-Positive Patients with Community-Acquired Pneumonia
- Other Laboratory Tests for Community-Acquired Pneumonia
- Chest Radiography and CT Scanning
- Fine-Needle Aspiration and Bronchoscopy With Bronchoalveolar Lavage
- Histologic Findings
- Pharmacologic Therapy
- Severity
- Outpatient Care in Community-Acquired Pneumonia
- Vaccination
- Infectious Diseases Society of America/American Thoracic Society CAP Guidelines
- Patient Instructions
- Acknowledgments
- Questions & Answers
- Show All
- Media Gallery
- References