Practice Essentials
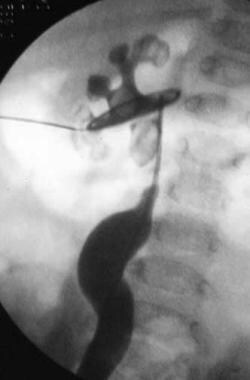
Percutaneous surgery is based on needle and guidewire access to the kidney and the upper urinary tract. (See the image below.) Once guidewire access is obtained, various catheters can then be placed into the kidney, either for drainage or for facilitation of antegrade intrarenal or ureteral endoscopic procedures. The tract must first be established and should provide a straightforward route to the kidney, allowing bloodless instrumentation. If more than renal drainage is desired, endoscopic surgery can then continue as a single or staged procedure. With recent advances, many invasive open urologic procedures are now performed endoscopically through small-diameter percutaneous access tracts.
History of the Procedure
The first percutaneous nephrostomy description of record was published in 1865 by Thomas Hillier, MD. Dr. Hillier, a young physician in London, repeatedly drained a 4-year-old boy's kidney, which he believed at that time to be congenitally obstructed. He performed multiple nephrostomies from 1863-1868 in an attempt to relieve the patient's abdominal distention, hoping to create a permanent fistula to the skin. This child ultimately died 5 years later, after persistent tapping, due to severe abdominal distention that led to ambulation and health complications. He was found to have a severely hydronephrotic kidney, as shown below, upon pathologic review. None of Hillier's contemporaries followed his example, and percutaneous renal procedures received no further attention until the mid 1950s.
See the image below.
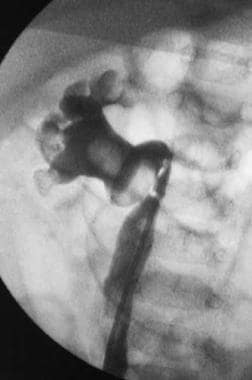 Retrograde ureteropyelogram that demonstrates a severely hydronephrotic kidney with multiple large intrarenal and ureteral calculi.
Retrograde ureteropyelogram that demonstrates a severely hydronephrotic kidney with multiple large intrarenal and ureteral calculi.
Goodwin and associates provided the next description of percutaneous renal access in 1955. [1, 2] They reported their experience with therapeutic percutaneous nephrostomies in 16 patients. In 1976, Fernstrom and Johansson described a procedure in which a renal pelvic calculus could be extracted through a percutaneous tract. [3]
Later, Blandy and Singh (1976) published important data, affirming that aggressive surgical management was necessary when treating staghorn calculus disease. They reported a high 10-year mortality rate (28%) with conservative treatment, compared with a 10-year mortality rate of 7.2% following surgical removal of the stone. In 2005, the clinical practice guideline report for the management of staghorn calculi by the American Urological Association guidelines panel confirmed that percutaneous treatment of staghorn calculi should be first-line treatment for most patients. [4]
The technology and techniques involved in percutaneous renal access and percutaneous surgery have evolved rapidly over the last 30 years. Percutaneous procedures are now performed on a routine basis for both diagnostic and therapeutic procedures. The modality of percutaneous surgery is evolving continuously as the endoscopes, radiography, and complementary instruments continue to improve.
Indications
Percutaneous access to the renal collecting system may be performed for diagnostic procedures or for establishing a tract through which therapeutic interventions and endoscopy are performed.
Diagnostic indications
Antegrade pyelography refers to the administration of radiopaque contrast material into the kidney and collecting system via a percutaneously placed needle or catheter system. It is rarely performed alone because of the current availability of other less-invasive radiographic techniques (eg, intravenous urography, ultrasonography, magnetic resonance imaging, computed tomography, retrograde studies) but is a common step toward other more complex endoscopic interventions. During most percutaneous-access procedures to the kidney, routine antegrade pyelography is performed. This often provides important information about the intrarenal collecting system architecture and can help plan subsequent therapeutic interventions.
Pressure/perfusion study (Whitaker test) is similar to an antegrade nephrostography but adds an in-line manometer to measure intrarenal collecting system pressures while filling and emptying. This test has largely been replaced by noninvasive nuclear medicine examinations of the kidney (eg, diuretic renography or scintigraphy [renal scan]).
However, in cases in which the diuretic renal scan findings are equivocal, a Whitaker test may be performed. During this test, dilute contrast is instilled at a rate of 10 mL/min via a percutaneously placed needle or small nephrostomy tube in the renal collecting system. The collecting system architecture is reviewed as the upper urinary tract is filled and drained of contrast. A urethral catheter allows drainage of the bladder and measurements of bladder pressures. With the kidney being perfused in an antegrade fashion at a flow rate of 10 mL/min, differential pressures between the renal pelvis and bladder of less than 13 cm of water are normal (not obstructed), 14-22 cm of water suggest mild obstruction, and more than 22 cm of water suggests moderate-to-severe ureteral obstruction.
Therapeutic indications
Most of the following therapeutic interventions were once performed via an open approach. With percutaneous renal access, these interventions carry significantly decreased morbidity than they did with open surgery. Percutaneous renal access is a minimally invasive technique that allows the patient quicker recovery time, better cosmetic effect, and a shorter hospital stay.
Nephrostomy catheter drainage
When retrograde drainage of the kidney is not indicated, is technically difficult (eg, impacted stone, tumor, stricture), or is not advisable (eg, ureteral obstruction, sepsis) and when drainage of the kidney is paramount, the kidneys are drained percutaneously. This is performed with either ultrasonography or fluoroscopic localization of the collecting system. In this setting, the surgeon's goal is to obtain drainage, not to perform other more complex therapeutic maneuvers. Small-diameter 8-12F catheters are placed over the access guidewire to obtain drainage of the upper urinary tract. This allows preservation of renal function, relief of symptoms, and effective and rapid drainage of purulent material from an obstructed kidney.
Antegrade ureteral stenting
A ureteral stent is a catheter that is placed into the ureter to facilitate internal drainage of the upper urinary tract. Most commonly, it is placed cystoscopically through the bladder. When retrograde stenting of the ureter is unsuccessful, the antegrade percutaneous approach is used. In this setting, percutaneous guidewire access is obtained first. Then, the guidewire and catheter are directed down the ureter and into the bladder fluoroscopically. If stenting is not possible cystoscopically due to ureteral tortuosity, false passages, or the inability to identify the orifice endoscopically, then the antegrade approach is frequently successful.
Treatment of ureteral strictures
Ureteral strictures are treated with balloon dilation and/or endoscopic incision. Endoscopic incisions are performed with endoscopes placed transurethrally/ureteroscopically or through a percutaneous renal access tract. Strictures are caused by iatrogenic injuries, gynecologic diseases such as pelvic endometriosis, or prior urinary tract surgeries. Dilation therapy alone for ureteral strictures has the lowest overall treatment success rate (30%), while endoscopic incisions produce better results. However, results can vary based on the length of the strictured segment, the extent of periureteral and retroperitoneal fibrosis, and a history of radiation therapy.
Percutaneous endopyelotomy
Similar to ureteral strictures, this refers to the endoscopic treatment of an obstructed ureteropelvic junction (UPJ). [5] This disorder is either primary (ie, congenital) or secondary (ie, due to chronic irritation from stone disease). In a study by Cyphers et al, the most common indication for percutaneous nephrostomy in neonates and young infants was ureteropelvic junction obstruction. [6]
Incision of the UPJ can be performed ureteroscopically or via a percutaneous access tract. Success rates are similar in both groups, but percutaneous treatment is particularly useful in complex cases in which associated intrarenal calculi are present, as ahown below. The percutaneous removal of these stones is essential when planning endopyelotomy because stone fragments that remain can migrate into the incision and be associated with a granulomatous reaction and subsequent obstruction. Antegrade endopyelotomy success rates range from 72-87% with up to 3 years of follow-up.
See the image below.
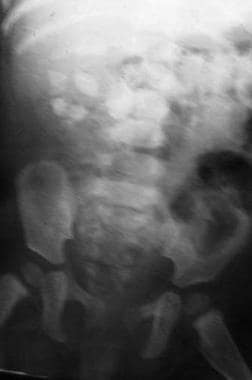 The patient is a 10-month-old girl with a history of fever and recurrent urinary tract infections. Plain radiograph demonstrates multiple large renal calculi.
The patient is a 10-month-old girl with a history of fever and recurrent urinary tract infections. Plain radiograph demonstrates multiple large renal calculi.
Various minimally invasive procedures have recently emerged for the treatment of UPJ obstruction. These include laparoscopic pyeloplasty and percutaneous endopyeloplasty. However, in appropriately selected patients, percutaneous endopyelotomy remains an appropriate and effective treatment option. Studies suggest that the success rate of endopyelotomy is decreased when a crossing vessel is the primary cause of UPJ obstruction, when the renal function is poor, or when severe hydronephrosis or a long segment (>2 cm) of ureteral obstruction is present. In these situations, open or laparoscopic pyeloplasty may be more appropriate.
When percutaneous endopyelotomy is performed, access to the kidney is established and a guidewire is passed across the UPJ. A full-thickness incision is then made via the UPJ with a cold-knife, electrocautery, or laser. An indwelling ureteral stent is placed for 3-5 weeks, and a nephrostomy tube is often left for 24-48 hours postoperatively.
Percutaneous endopyeloplasty
Endopyeloplasty has emerged as a viable option that combines antegrade endopyelotomy with endoscopic suturing, like that used with laparoscopic pyeloplasty. Some centers are now using endopyeloplasty.
Percutaneous endopyeloplasty, as described by Gill et al [7] (2002) and Desai et al [8] (2004), consists of retrograde ureteral catheterization, percutaneous renal access, longitudinal endopyelotomy incision using a cone-tip Bugbee electrode and cutting current, mobilization of the distal ureter, percutaneous horizontal suturing vertically of the endopyelotomy incision, and then antegrade placement of a double-J ureteral stent and nephrostomy tube. Suturing was performed using a modified 5-mm laparoscopic suturing device (SewRight SR5, LSI Solutions, Victor, NY) inserted through the working channel of the nephroscope.
Although results show percutaneous endopyeloplasty is technically feasible and safe, this procedure carries significant limitations. Relative contraindications include long-segment stenosis, extrinsic crossing vessels, and prior surgery for UPJ obstruction. In addition, long-term data and additional studies from multicenter institutions with larger patient groups and longer follow-up are necessary to make percutaneous endopyeloplasty a first-line treatment option for primary UPJ obstruction.
Percutaneous nephrolithotomy
Certain upper urinary tract stones that are not amenable to other modalities of treatment (eg, shockwave lithotripsy, retrograde ureteroscopic treatment) are frequently treated via this percutaneous endoscopic approach. [9] Commonly, infected stone burdens are treated percutaneously because this facilitates not only stone fragmentation but also prompt evacuation of the infected material. Often, initial percutaneous drainage of an infected system with a stone is needed, allowing for a cooling-down period prior to definitive therapeutic interventions. Large stones (>2.5 cm in diameter), including staghorn calculi, should be treated via percutaneous endoscopic surgery. Open surgery is performed only for a small minority of staghorn calculi that would require an excessive number of percutaneous access tracts to clear the entire stone burden.
Another indication for percutaneous nephrolithotomy (PCNL) is symptomatic calculi located in a caliceal diverticulum (dilated cavities connected to the collecting system by a narrow infundibulum). In this situation, percutaneous treatment results in 88-100% stone-free rates and symptom-free rates. Large stone burdens or those within a long-necked diverticulum are best treated in this fashion.
Traditionally, PCNL has been performed in the prone position and is an established technique with a high success rate a low morbidity. [10] However, the prone position can be challenging and is associated with high risk in morbidly obese patients with cardiopulmonary disease; thus, the supine position has been established to help overcome these drawbacks. Theoretical advantages of the supine position include safer and easier positioning for the patient, less cardiovascular change and risk, no need for patient reposition, and possible shorter operating time.
Theoretical disadvantages of the supine position include anterior/lateral puncture sites, potentially increasing the risk for visceral organ injury (eg, colon, liver, spleen); collapse of the collecting system; and difficulty with approaching the upper pole calyx. [11]
Liu et al performed a systematic review and meta-analysis of percutaneous nephrolithotomy for patients in the supine versus prone position and found that PCNL in the supine position was as safe and efficacious as the conventional prone position; however, no overwhelming evidence suggested one position was better then the other. [12]
Although supine PCNL has become a option for the removal of large stone burdens, additional prospective, randomized controlled studies are needed to assess the safety and long term efficacy of this approach. Surgeon preference and experience should ultimately guide the choice of patient positioning during PCNL.
A Cochrane review showed that the use of tranexamic acid may reduce blood transfusions and major surgical complications, and possibly increase the stone-free rate, in patients undergoing PCNL. However, it may also increase adverse events, and its effects on secondary interventions, minor surgical complications, unplanned hospitalizations or readmissions, and hospital length of stay are uncertain. [13]
Another Cochrane review found that PCNL may increase stone-free rates and reduce the need for secondary interventions compared with retrograde intrarenal surgery, but with no significant effect on major complications or ureteral strictures. Moreover, PCNL may extend the length of hospital stay. [14]
Miniature percutaneous nephrolithotomy (Mini-Perc)
Miniature percutaneous nephrolithotomy (Mini-Perc) was originally described by Helal et al as a means of extracting renal stones refractory to ESWL in a 2-year old premature child. The method described by Helal et al involved dilating sequentially to a 16F sheath followed by use of a 12F vascular peel-away sheath. A pediatric endoscope was used to extract stones. [15] Jackman et al modified the Mini-Perc technique to use an 11F ureteral access sheath. In their series of 9 cases, the stone-free rate was 89% at a follow-up of 3.8 weeks. [16]
Proponents of the Mini-Perc procedure cite limited blood loss, increased maneuverability, decreased postoperative pain, and a limited hospital stay. Limitations of the procedure include having to fragment stones into small enough pieces to fit through a reduced-sized sheath and longer operative times. [17]
Mishra et al conducted a prospective study comparing Mini-Perc with standard PCNL for stones 1-2 cm in size. [18] The Mini-Perc group used an 18F sheath (range 15-20F) compared with a 26.8F sheath (range 24-30F) in the standard PCNL group. The study concluded that the Mini-Perc technique had longer operative times (45 vs 31 min), lower blood loss (0.8 vs 1.3 g hemoglobin), and a shorter hospital stay (3.2 vs 4.8 d). The postoperative stone-free rates were similar between the 2 groups at a follow-up of 1 month.
Additionally, Cheng et al conducted a randomized trial comparing Mini-Perc to standard PCNL. They concluded that the Mini-Perc was associated with longer operative times (134 vs 89 min) secondary to a smaller sheath; however, blood loss necessitating transfusion was statistically significantly lower (1.4% vs 10.4%). Stone-free rates for staghorn and simple renal pelvic stones were similar between the 2 groups; however, the Mini-Perc group had a statistically significantly higher stone-free rate with multiple calyceal stones. Hospital stay, postoperative pain, and stone clearance rates were similar in the 2 groups. [19]
Tubeless percutaneous nephrolithotomy
Tubeless PCNL refers to the technique of performing a nephroscopic procedure via a fresh access track without subsequent placement of a nephrostomy tube.
Earlier techniques used an internal ureteral stent for drainage [20] ; however, more recent studies have focused on totally tubeless drainage in which neither an external nephrostomy nor an internal ureteral stent are left after the procedure. [21] Benefits of the tubeless and totally tubeless techniques include reduction in postoperative pain and a shorter hospital course. [22, 23] Modifications of the totally tubeless technique are the use of hemostatic agents such as fibrin glue injections to help seal the nephrostomy tract following PCNL. [24] Wang et al performed a meta-analysis of 7 studies and concluded that tubeless PCNL offers the advantage of reduced hospital stay and need for postoperative analgesia in select, uncomplicated cases. There were no differences in operation duration or hematocrit change between the 2 groups. [25]
Although tubeless PCNL has been shown to be feasible and to reduce postoperative pain, it is contraindicated in complicated cases (eg, bleeding, renal perforation, extravasation, infection) or if a staged PCNL is necessary. Additional studies are needed to demonstrate the clear clinical benefit of tubeless PCNL.
Perfusion chemolysis to dissolve and clear certain renal stones
This therapy is now largely adjunctive in nature owing to the advent of new lithotripsy techniques and oral chemolytic agents (eg, potassium citrate). Chemolysis of uric acid and cystine stones is particularly effective. Bicarbonate solution produces adequate alkalinization to dissolve uric acid debris while a combination of tris- (hydroxymethylene)-aminomethane (THAM) and Mucomyst (N -acetylcysteine) is used for cystine stones. Following nephrolithotomy, percutaneous catheters are left in the collecting system and used to instill and drain irrigant that is used to dissolve and flush residual fragments.
To limit risks, a double-catheter system should be used. This enables simultaneous instillation and drainage. A combination of catheters should be used. One may use 2 separately placed nephrostomy catheters or a ureteral catheter in conjunction with a nephrostomy tube. The irrigation catheter should be placed close to the stone to ensure adequate exposure of the stone to the irrigant. One should not use perfusion therapy in the presence of infected urine because this may result in sepsis. A flow rate of 100-120 mL/h is standard. An in-line manometer is useful for measuring and maintaining low (< 20 cm of water) intrarenal pressures. This is particularly useful when irrigants such as Renacidin are used to dissolve infectious matrix debris. The time necessary for stone dissolution depends on the stone burden and composition. Irrigations may dissolve in a few days (ie, with uric acid) or may require weeks (ie, with cystine or struvite). As a rule, 1 cm per month can be expected.
The irrigants that may be used include potassium or sodium bicarbonate for uric acid stones; D-penicillamine, N -acetylcysteine, or tromethamine-E solution for cystine stones; or Suby solution (G or M) or hemiacidrin (Renacidin) for struvite or apatite stones.
Renacidin irrigant is used specifically for infectious matrix stone burdens and can be useful in clearing residual debris after PCNL. Care must be taken when using this irrigant because high intrarenal pressures may cause inadvertent vascular absorption with subsequent hypermagnesemia. An in-line manometer and careful attention to maintaining low intrarenal pressures (< 20 cm of water) help prevent this complication.
Endoscopic resection and treatment of upper urinary tract urothelial tumors
Conventional treatment of upper tract urothelial lesions is based on radical open surgery, ie, nephroureterectomy and resection of a bladder cuff or segmental ureterectomy. In patients with severe comorbidities, bilateral tumors, or solitary kidneys, renal-sparing surgery is an option. Endoscopic treatment is performed ureteroscopically or through a percutaneous tract. Electrocautery and/or laser energy is used for tumor resection and/or coagulation in a fashion similar to that of standard transurethral resection of bladder tumors. Postoperatively, topical chemotherapy (ie, mitomycin-C, thiotepa) may be instilled via a nephrostomy tube or ureteral catheter to reduce recurrence rates. Although safe, confirmation of the effectiveness of this immediate adjuvant topical therapy awaits the results of large trials.
The retrograde ureteroscopic approach is favored over percutaneous endoscopic therapy because it is less invasive and avoids the possibilities of percutaneous tract seeding. In select cases with large tumor burdens, a mature nephrostomy tract may help facilitate clearance. Success with both forms of treatment is based on the presenting tumor grade and stage. Low-grade urothelial tumors are associated with the best outcomes with these treatments. Close life-long endoscopic surveillance is required to detect any recurrences.
Relevant Anatomy
The kidneys are retroperitoneal organs that are obliquely placed as they lie on the anterior surface of the psoas muscles. The right kidney is often 2-8 cm lower than the left due to the presence of the liver on the right side. It lies adjacent to the 12th rib, liver, duodenum, and hepatic flexure of the colon. The left kidney is adjacent to the 11th and 12th ribs, pancreas, spleen, and splenic flexure of the colon.
The kidney may change location with changes in patient position and respiration. Normally, kidneys do not exceed 8-9 cm excursion when a patient stands from a supine position. With the respiratory cycle, the kidneys naturally move in a vertical direction. Due to the close proximity of the kidney to the pleural cavity, a risk of pleurotomy is incurred with a percutaneous puncture, especially during upper-pole renal access. To help prevent pneumothorax, percutaneous renal access above the 12th rib should be performed near the end of the rib.
The collecting system of the kidney is composed of minor calices (2-4), major calices, and a renal pelvis. The renal pelvis lies in the renal sinus and narrows at the UPJ to form the ureter. In the renal hilum, the vein is usually situated anterior and the renal pelvis is posterior, with the artery lying in between.
Contraindications
The only contraindication to percutaneous renal access is an uncorrected coagulopathy. Correcting any abnormalities prior to percutaneous access is essential. Thrombocytopenia should be corrected with platelet administration. For elective procedures, patients on warfarin (Coumadin) should have their coagulation factors normalized prior to surgery.
-
The patient is a 10-month-old girl with a history of fever and recurrent urinary tract infections. Plain radiograph demonstrates multiple large renal calculi.
-
Retrograde ureteropyelogram that demonstrates a severely hydronephrotic kidney with multiple large intrarenal and ureteral calculi.
-
Initial percutaneous renal access obtained via a lower-pole puncture.
-
After initial needle access is obtained, a guidewire is coiled in the renal pelvis prior to tract dilation.
-
After tract dilation, the calculi are treated with a rigid nephroscope and ultrasonic lithotriptor. A 7.5F flexible nephroscope and a laser lithotriptor are used to treat stones in tangential calyces. All aspects of the intrarenal collecting system are accessible, which requires both primary and secondary deflection.
-
Following treatment of the large stone burden, the remainder of the collecting system is examined in an antegrade manner via flexible ureteroscopy.
-
Following the procedure, a 14F Malecot nephrostomy tube and a 5F nephroureteral stent are placed for renal drainage.
-
Percutaneous nephrolithotomy. Video courtesy of Dennis G Lusaya, MD, and Edgar V Lerma, MD.