Practice Essentials
Deep venous thrombosis (DVT) is a manifestation of venous thromboembolism (VTE). Although most DVT is occult and resolves spontaneously without complication, death from DVT-associated massive pulmonary embolism (PE) causes as many as 300,000 deaths annually in the United States. [1] See the image below.
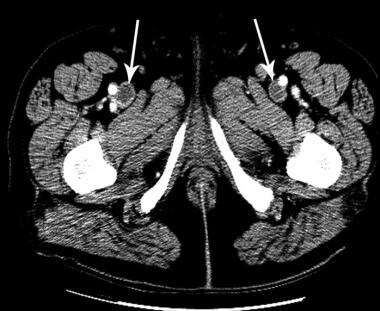 Deep Venous Thrombosis (DVT). The computed tomography venogram shows bilateral deep venous thrombosis (arrows).
Deep Venous Thrombosis (DVT). The computed tomography venogram shows bilateral deep venous thrombosis (arrows).
Signs and symptoms
Symptoms of DVT may include the following:
-
Edema - Most specific symptom
-
Leg pain - Occurs in 50% of patients but is nonspecific
-
Tenderness - Occurs in 75% of patients
-
Warmth or erythema of the skin over the area of thrombosis
-
Clinical symptoms of PE as the primary manifestation
As many as 46% with patients with classic symptoms have negative venograms, [2] and as many as 50% of those with image-documented venous thrombosis lack specific symptoms. [2, 3]
No single physical finding or combination of symptoms and signs is sufficiently accurate to establish the diagnosis of DVT, but physical findings in DVT may include the following:
-
Calf pain on dorsiflexion of the foot (Homans sign)
-
A palpable, indurated, cordlike, tender subcutaneous venous segment
-
Variable discoloration of the lower extremity
-
Blanched appearance of the leg because of edema (relatively rare)
Potential complications of DVT include the following:
-
As many as 40% of patients have silent PE when symptomatic DVT is diagnosed [4]
-
Paradoxic emboli (rare)
-
Recurrent DVT
-
Postthrombotic syndrome (PTS)
See Clinical Presentation for more detail.
Diagnosis
The American Academy of Family Physicians (AAFP)/American College of Physicians (ACP) recommendations for workup of patients with probable DVT are as follows [5] :
-
Validated clinical prediction rules (eg, Wells) should be used to estimate the pretest probability of VTE and interpret test results
-
In appropriately selected patients with low pretest probability of DVT or PE, it is reasonable to obtain a high-sensitivity D-dimer
-
In patients with intermediate to high pretest probability of lower-extremity DVT, ultrasonography is recommended
-
In patients with intermediate or high pretest probability of PE, diagnostic imaging studies (eg, ventilation-perfusion scan, multidetector helical CT, and pulmonary angiography) are required
The main laboratory studies to be considered include the following:
-
D-dimer testing
-
Coagulation studies (eg, prothrombin time and activated partial thromboplastin time) to evaluate for a hypercoagulable state
See Workup for more detail.
Management
Treatment options for DVT include the following:
-
Anticoagulation (mainstay of therapy) - Heparins, warfarin, factor Xa inhibitors, and various emerging anticoagulants
-
Pharmacologic thrombolysis
-
Endovascular and surgical interventions
-
Physical measures (eg, elastic compression stockings and ambulation)
Heparin products used in the treatment of DVT include the following:
-
Low-molecular-weight heparin (LMWH; eg, enoxaparin)
-
Unfractionated heparin (UFH)
Factor Xa inhibitors used in the treatment of DVT include the following:
-
Fondaparinux – This agent appears to be comparable to enoxaparin with respect to efficacy and safety [6]
Endovascular therapy is performed to reduce the severity and duration of lower-extremity symptoms, prevent PE, diminish the risk of recurrent VTE, and prevent PTS. Percutaneous transcatheter treatment of DVT includes the following:
-
Thrombus removal with catheter-directed thrombolysis – American College of Chest Physicians (ACCP) recommends thrombolytic therapy only for patients with massive iliofemoral vein thrombosis associated with limb ischemia or vascular compromise
-
Mechanical thrombectomy
-
Angioplasty
-
Stenting of venous obstructions
American Heart Association (AHA) recommendations for inferior vena cava filters include the following [10] :
-
Confirmed acute proximal DVT or acute PE in patients contraindicated for anticoagulation
-
Recurrent thromboembolism while on anticoagulation
-
Active bleeding complications requiring termination of anticoagulation therapy
See Treatment and Medication for more detail.
Background
Deep venous thrombosis (DVT) and pulmonary embolism (PE) are manifestations of a single disease entity, namely, venous thromboembolism (VTE). The earliest known reference to peripheral venous disease is found on the Eber papyrus, which dates from 1550 BC and documents the potentially fatal hemorrhage that may ensue from surgery on varicose veins. In 1644, Schenk first observed venous thrombosis when he described an occlusion in the inferior vena cava. In 1846, Virchow recognized the association between venous thrombosis in the legs and PE.
DVT is the presence of coagulated blood, a thrombus, in one of the deep venous conduits that return blood to the heart. The clinical conundrum is that symptoms (pain and swelling) are often nonspecific or absent. However, if left untreated, the thrombus may become fragmented or dislodged and migrate to obstruct the arterial supply to the lung, causing potentially life-threatening PE See the images below.
DVT most commonly involves the deep veins of the leg or arm, often resulting in potentially life-threatening emboli to the lungs or debilitating valvular dysfunction and chronic leg swelling. Over the past 25 years, the pathophysiology of DVT has become much better understood, and considerable progress has been made in its diagnosis and treatment.
DVT is one of the most prevalent medical problems today, with an annual incidence of 80 cases per 100,000. Each year in the United States, more than 200,000 people develop venous thrombosis; of those, 50,000 cases are complicated by PE. [11] Lower-extremity DVT is the most common venous thrombosis, with a prevalence of 1 case per 1000 population. In addition, it is the underlying source of 90% of acute PEs, which cause 25,000 deaths per year in the United States (National Center for Health Statistics [NCHS], 2006).
Conclusive diagnosis has historically required invasive and expensive venography, which is still considered the criterion standard. The diagnosis may also be obtained noninvasively by means of ultrasonographic examination. (See Workup.)
Early recognition and appropriate treatment of DVT and its complications can save many lives. (See Treatment and Management.) The goals of pharmacotherapy for DVT are to reduce morbidity, prevent postthrombotic syndrome (PTS), and prevent PE. The primary agents include anticoagulants and thrombolytics. (See Medication.)
Other than the immediate threat of PE, the risk of long-term major disability from postthrombotic syndrome is high. [12, 13, 14, 15, 16]
For patient education resources, see the Lung Disease & Respiratory Health Center, as well as the patient education articles Deep Vein Thrombosis (Blood Clot in the Leg, DVT), Phlebitis, and Pulmonary Embolism.
Anatomy
The peripheral venous system functions both as a reservoir to hold extra blood and as a conduit to return blood from the periphery to the heart and lungs. Unlike arteries, which possess 3 well-defined layers (a thin intima, a well-developed muscular media, and a fibrous adventitia), most veins are composed of a single tissue layer. Only the largest veins possess internal elastic membranes, and this layer is thin and unevenly distributed, providing little buttress against high internal pressures. The correct functioning of the venous system depends on a complex series of valves and pumps that are individually frail and prone to malfunction, yet the system as a whole performs remarkably well under extremely adverse conditions.
Primary collecting veins of the lower extremity are passive, thin-walled reservoirs that are tremendously distensible. Most are suprafascial, surrounded by loosely bound alveolar and fatty tissue that is easily displaced. These suprafascial collecting veins can dilate to accommodate large volumes of blood with little increase in back pressure so that the volume of blood sequestered within the venous system at any moment can vary by a factor of 2 or more without interfering with the normal function of the veins. Suprafascial collecting veins belong to the superficial venous system.
Outflow from collecting veins is via secondary conduit veins that have thicker walls and are less distensible. Most of these veins are subfascial and are surrounded by tissues that are dense and tightly bound. These subfascial veins belong to the deep venous system, through which all venous blood must eventually pass through on its way back to the right atrium of the heart. The lower limb deep venous system is typically thought of as 2 separate systems, one below the knee and one above.
The calf has 3 groups of paired deep veins: the anterior tibial veins, draining the dorsum of the foot; the posterior tibial veins, draining the sole of the foot; and the peroneal veins, draining the lateral aspect of the foot. Venous sinusoids within the calf muscle coalesce to form soleal and gastrocnemius intramuscular venous plexuses, which join the peroneal veins in the mid calf. These veins play an important role in the muscle pump function of the calf. Just below the knee, these tibial veins join to become the popliteal vein, which too can be paired on occasion.
Together, the calf’s muscles and deep vein system form a complex array of valves and pumps, often referred to as the “peripheral heart,” that functions to push blood upward from the feet against gravity. The calf-muscle pump is analogous to the common hand-pump bulb of a sphygmomanometer filling a blood pressure cuff. Before pumping has started, the pressure is neutral and equal everywhere throughout the system and the calf fills with blood, typically 100-150 mL. When the calf contracts, the feeding perforator vein valves are forced closed and the outflow valves are forced open driving the blood proximally. When the calf is allowed to relax, the veins and sinusoids refill from the superficial venous system via perforating veins, and the outflow valve is then forced shut, preventing retrograde flow. With each “contraction,” 40-60% of the calf’s venous volume is driven proximally. [17]
The deep veins of the thigh begin distally with the popliteal vein as it courses proximally behind the knee and then passes through the adductor canal, at which point its name changes to the femoral vein. (This important deep vein is sometimes incorrectly referred to as the superficial femoral vein in a misguided attempt to distinguish it from the profunda femoris, or deep femoral vein, a short, stubby vein that usually has its origin in terminal muscle tributaries within the deep muscles of the lateral thigh but may communicate with the popliteal vein in up to 10% of patients.
The term superficial femoral vein should never be used, because the femoral vein is in fact a deep vein and is not part of the superficial venous system. This incorrect term does not appear in any definitive anatomic atlas, yet it has come into common use in vascular laboratory practice. Confusion arising from use of the inappropriate name has been responsible for many cases of clinical mismanagement and death.) In theproximal thigh,the femoral vein and the deep femoral vein unite to form the common femoral vein, which passes upwards above the groin crease to become the iliac vein.
The external iliac vein is the continuation of the femoral vein as it passes upward behind the inguinal ligament. At the level of the sacroiliac joint, it unites with the hypogastric vein to form the common iliac vein. The left common iliac is longer than the right and more oblique in its course, passing behind the right common iliac artery. This anatomic asymmetry sometimes results in compression of the left common iliac vein by the right common iliac artery to produce May-Thurner syndrome, a left-sided iliac outflow obstruction with localized adventitial fibrosis and intimal proliferation, often with associated deep venous thrombosis. At the level of the fifth lumbar vertebra, the 2 common iliac veins come together at an acute angle to form the inferior vena cava.
Please go to the main article on Inferior Vena Caval Thrombosis for more information.
Pathophysiology
Over a century ago, Rudolf Virchow described 3 factors that are critically important in the development of venous thrombosis: (1) venous stasis, (2) activation of blood coagulation, and (3) vein damage. These factors have come to be known as the Virchow triad.
Venous stasis can occur as a result of anything that slows or obstructs the flow of venous blood. This results in an increase in viscosity and the formation of microthrombi, which are not washed away by fluid movement; the thrombus that forms may then grow and propagate. Endothelial (intimal) damage in the blood vessel may be intrinsic or secondary to external trauma. It may result from accidental injury or surgical insult. A hypercoagulable state can occur due to a biochemical imbalance between circulating factors. This may result from an increase in circulating tissue activation factor, combined with a decrease in circulating plasma antithrombin and fibrinolysins.
Over time, refinements have been made in the description of these factors and their relative importance to the development of venous thrombosis. The origin of venous thrombosis is frequently multifactorial, with components of the Virchow triad assuming variable importance in individual patients, but the end result is early thrombus interaction with the endothelium. This interaction stimulates local cytokine production and facilitates leukocyte adhesion to the endothelium, both of which promote venous thrombosis. Depending on the relative balance between activated coagulation and thrombolysis, thrombus propagation occurs.
Decreased vein wall contractility and vein valve dysfunction contribute to the development of chronic venous insufficiency. The rise in ambulatory venous pressure causes a variety of clinical symptoms of varicose veins, lower extremity edema, and venous ulceration.
Development of thrombosis
Thrombosis is the homeostatic mechanism whereby blood coagulates or clots, a process crucial to the establishment of hemostasis after a wound. It may be initiated via several pathways, usually consisting of cascading activation of enzymes that magnify the effect of an initial trigger event. A similar complex of events results in fibrinolysis, or the dissolution of thrombi. The balance of trigger factors and enzymes is complex. Microscopic thrombus formation and thrombolysis (dissolution) are continuous events, but with increased stasis, procoagulant factors, or endothelial injury, the coagulation-fibrinolysis balance may favor the pathologic formation of an obstructive thrombus. Clinically relevant deep venous thrombosis is the persistent formation of macroscopic thrombus in the deep proximal veins.
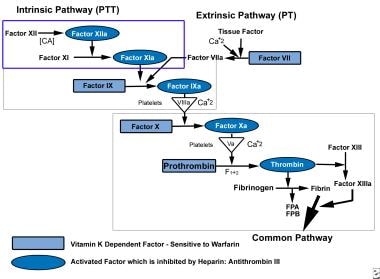
For the most part, the coagulation mechanism consists of a series of self-regulating steps that result in the production of a fibrin clot. These steps are controlled by a number of relatively inactive cofactors or zymogens, which, when activated, promote or accelerate the clotting process. These reactions usually occur at the phospholipid surface of platelets, endothelial cells, or macrophages. Generally, the initiation of the coagulation process can be divided into 2 distinct pathways, an intrinsic system and an extrinsic system (see the image below).
The extrinsic system operates as the result of activation by tissue lipoprotein, usually released as the result of some mechanical injury or trauma. The intrinsic system usually involves circulating plasma factors. Both of these pathways come together at the level of factor X, which is activated to form factor Xa. This in turn promotes the conversion of prothrombin to thrombin (factor II). This is the key step in clot formation, for active thrombin is necessary for the transformation of fibrinogen to a fibrin clot.
Once a fibrin clot is formed and has performed its function of hemostasis, mechanisms exist in the body to restore the normal blood flow by lysing the fibrin deposit. Circulating fibrinolysins perform this function. Plasmin digests fibrin and also inactivates clotting factors V and VIII and fibrinogen.
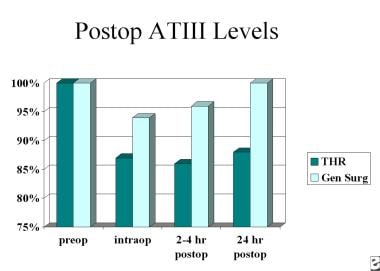
Three naturally occurring anticoagulant mechanisms exist to prevent inadvertent activation of the clotting process. These include the heparin-antithrombin III (ATIII), protein C and thrombomodulin protein S, and the tissue factor inhibition pathways. When trauma occurs, or when surgery is performed, circulating ATIII is decreased. This has the effect of potentiating the coagulation process. Studies have demonstrated that levels of circulating ATIII is decreased more, and stay reduced longer, after total hip replacement (THR) than after general surgical cases (see the image below).
Furthermore, patients who have positive venograms postoperatively tend to be those in whom circulating levels of ATIII are diminished (see the image below).
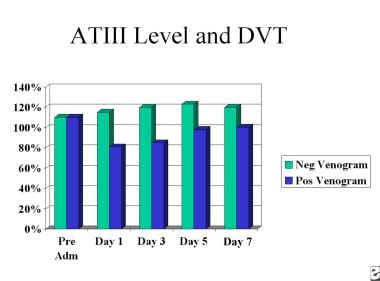 Deep Venous Thrombosis (DVT). This chart depicts perioperative antithrombin III levels and DVT formation.
Deep Venous Thrombosis (DVT). This chart depicts perioperative antithrombin III levels and DVT formation.
Under normal circumstances, a physiologic balance is present between factors that promote and retard coagulation. A disturbance in this equilibrium may result in the coagulation process occurring at an inopportune time or location or in an excessive manor. Alternatively, failure of the normal coagulation mechanisms may lead to hemorrhage.
Thrombus usually forms behind valve cusps or at venous branch points, most of which begin in the calf. Venodilation may disrupt the endothelial cell barrier and expose the subendothelium. Platelets adhere to the subendothelial surface by means of von Willebrand factor or fibrinogen in the vessel wall. Neutrophils and platelets are activated, releasing procoagulant and inflammatory mediators. Neutrophils also adhere to the basement membrane and migrate into the subendothelium. Complexes form of the surface of platelets and increase the rate of thrombin generation and fibrin formation. Stimulated leukocytes irreversibly bind to endothelial receptors and extravasate into the vein wall by means of mural chemotaxis. Because mature thrombus composed of platelets, leukocytes and fibrin develops, and an active thrombotic and inflammatory process occurs at the inner surface of the vein, and an active inflammatory response occurs in the wall of the vein. [18, 19]
Studies have shown that low flow sites, such as the soleal sinuses, behind venous valve pockets, and at venous confluences, are at most risk for the development of venous thrombi. [20, 21] However, stasis alone is not enough to facilitate the development of venous thrombosis. Experimental ligation of rabbit jugular veins for periods of up to 60 minutes have failed to consistently cause venous thrombosis. [22, 23] Although, patients that are immobilized for long periods of time seem to be at high risk for the development of venous thrombosis, an additional stimulus is required to develop deep venous thrombosis (DVT).
Evolution of venous insufficiency
Over time, thrombus organization begins with the infiltration of inflammatory cells into the clot. This results in a fibroelastic intimal thickening at the site of thrombus attachment in most patients and a fibrous synechiae in up to 11%. [24] In many patients, this interaction between vessel wall and thrombus leads to valvular dysfunction and overall vein wall fibrosis. Histological examination of vein wall remodeling after venous thrombosis has demonstrated an imbalance in connective tissue matrix regulation and a loss of regulatory venous contractility that contributes to the development of chronic venous insufficiency. [25, 26] Some form of chronic venous insufficiency develops in 29-79% of patients with an acute DVT, while ulceration is noted in 4-6%. [27, 28] The risk has been reported to be 6 times greater in those patients with recurrent thrombosis. [29]
Over a few months, most acute DVTs evolve to complete or partial recanalization, and collaterals develop (see the images below). [30, 31, 32, 33, 34, 35] Although blood flow may be restored, residual evidence of thrombus or stenosis is observed in half the patients after 1 year. Furthermore, the damage to the underlying valves and those compromised by peripheral dilation and insufficiency usually persists and may progress. Venous stasis, venous reflux, and chronic edema are common in patients who have had a large DVT. [36]
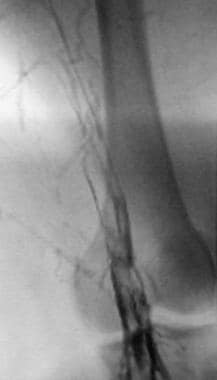 Deep Venous Thrombosis (DVT). This lower-extremity venogram shows outlining of an DVT in the popliteal vein with contrast enhancement.
Deep Venous Thrombosis (DVT). This lower-extremity venogram shows outlining of an DVT in the popliteal vein with contrast enhancement.
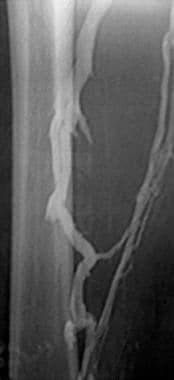 Deep Venous Thrombosis (DVT). The lower-extremity venogram reveals a nonocclusive chronic thrombus. The superficial femoral vein (lateral vein) has the appearance of two parallel veins, when in fact it is one lumen containing a chronic linear thrombus. Although the chronic clot is not obstructive after it recanalizes, it effectively causes the venous valves to adhere in an open position, predisposing the patient to reflux in the involved segment.
Deep Venous Thrombosis (DVT). The lower-extremity venogram reveals a nonocclusive chronic thrombus. The superficial femoral vein (lateral vein) has the appearance of two parallel veins, when in fact it is one lumen containing a chronic linear thrombus. Although the chronic clot is not obstructive after it recanalizes, it effectively causes the venous valves to adhere in an open position, predisposing the patient to reflux in the involved segment.
The acute effect of an occluded outflow vein may be minimal if adequate collateral pathways exist. As an alternative, it may produce marked pain and swelling if flow is forced retrograde. In the presence of deep vein outflow obstruction, contraction of the calf muscle produces dilation of the feeding perforating veins, it renders the valves nonfunctional (because the leaflets no longer coapt), and it forces the blood retrograde through the perforator branches and into the superficial system. This high-pressure flow may cause dilation of the superficial (usually low-pressure) system and produce superficial venous incompetence. In clinical terms, the increased incidence of reflux in the ipsilateral greater saphenous vein increases 8.7-fold on follow-up of DVT. [30] This chain of events (ie, obstruction to antegrade flow producing dilation, stasis, further valve dysfunction, with upstream increased pressure, dilation, and other processes) may produce hemodynamic findings of venous insufficiency.
Another mechanism that contributes to venous incompetence is the natural healing process of the thrombotic vein. The thrombotic mass is broken down over weeks to months by inflammatory reaction and fibrinolysis, and the valves and venous wall are altered by organization and ingrowth of smooth muscle cells and production of neointima. This process leaves damaged, incompetent, underlying valves, predisposing them to venous reflux. The mural inflammatory reaction breaks down collagen and elastin, leaving a noncompliant venous wall. [30, 31, 32, 33, 34, 35]
Persistent obstructive thrombus, coupled with valvular damage, ensures continuation of this cycle. Over time, the venous damage may become irreversible. Hemodynamic venous insufficiency is the underlying pathology of postthrombotic syndrome (PTS), also referred to as postphlebitic syndrome. If numerous valves are affected, flow does not occur centrally unless the leg is elevated. Inadequate expulsion of venous blood results in stasis and a persistently elevated venous pressure or venous hypertension. As fibrin extravasates and inflammation occurs, the superficial tissues become edematous and hyperpigmented. With progression, fibrosis compromises tissue oxygenation, and ulceration may result. After venous insufficiency occurs, no treatment is ideal; elevation and use of compression stockings may compensate, or surgical thrombectomy or venous bypass may be attempted. [37, 38, 39, 40]
With anticoagulation alone, as many as 75% of patients with symptomatic DVT present with PTS at 5-10 years. [40, 41] However, the incidence of venous ulceration is far less, at 5%. Of the half million patients with venous ulcers in the United States, 17-45% report having a history of DVT. [42]
Lower-extremity deep venous thrombosis
Most small thrombi in the lower extremities tend to resolve spontaneously after surgery. In about 15% of cases, however, these thrombi may extend into the proximal femoral venous system of the leg. Untreated proximal thrombi represent a significant source of clinically significant pulmonary emboli.
In the absence of rhythmic contraction of the leg muscles, as in walking or moving, blood flow in the veins slows and even stops in some areas, predisposing patients to thrombosis. [43]
In the postoperative patient, as many as one half of all isolated calf vein thrombi resolve spontaneously within a few hours, whereas approximately 15% extend to involve the femoral vein. A many as one third of untreated symptomatic calf vein DVT extend to the proximal veins. [44] At 1-month follow-up of untreated proximal DVT, 20% regress and 25% propagate. Although calf vein thrombi are rare sources of clinically significant pulmonary embolism (PE), the incidence of PE with untreated proximal thrombi is 29-50%. [44, 45] Most PEs are first diagnosed at autopsy. [46, 47]
Upper-extremity deep venous thrombosis
The 2 forms of upper-extremity DVT are (1) effort-induced thrombosis (Paget-von Schrötter syndrome) and (2) secondary thrombosis.
Effort induced thrombosis, or Paget-von Schrötter syndrome, accounts for 25% of cases. [48] Paget in England and von Schrötter in Germany independently described effort thrombosis more than 100 years ago. In this primary form of the disease, an underlying chronic venous compressive abnormality caused by the musculoskeletal structures in the costoclavicular space is present at the thoracic inlet and/or outlet. See the images below.
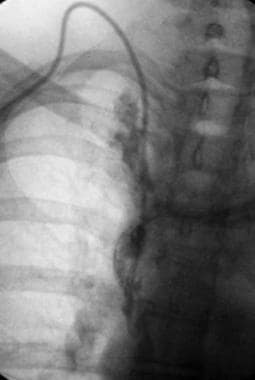 Deep Venous Thrombosis (DVT). This contrast-enhanced study was obtained through a Mediport placed through the chest wall through the internal jugular vein to facilitate chemotherapy. A thrombus has propagated peripherally from the tip of the catheter in the superior vena cava into both subclavian veins.
Deep Venous Thrombosis (DVT). This contrast-enhanced study was obtained through a Mediport placed through the chest wall through the internal jugular vein to facilitate chemotherapy. A thrombus has propagated peripherally from the tip of the catheter in the superior vena cava into both subclavian veins.
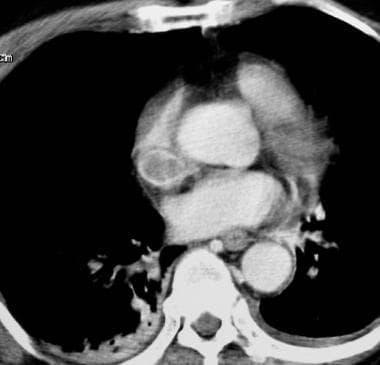 Deep Venous Thrombosis (DVT). Superior vena cava syndrome is noted in a patient with lung cancer. The computed tomography scan demonstrates a hypoattenuating thrombus that fills the superior vena cava. The patient was treated with anticoagulation alone.
Deep Venous Thrombosis (DVT). Superior vena cava syndrome is noted in a patient with lung cancer. The computed tomography scan demonstrates a hypoattenuating thrombus that fills the superior vena cava. The patient was treated with anticoagulation alone.
In 75% of patients with secondary thrombosis, hypercoagulability and/or indwelling central venous catheters are important contributing factors. In fact, with the advent of central venous catheters, upper-extremity and brachiocephalic venous thrombosis has become a more common problem. [49, 50, 51, 52]
For more information on upper-extremity DVT, see Imaging in Deep Venous Thrombosis of the Upper Extremity.
Pulmonary embolism
PE develops as venous thrombi break off from their location of origin and travel through the right heart and into the pulmonary artery, causing a ventilation perfusion defect and cardiac strain. PE occurs in approximately 10% of patients with acute DVT and can cause up to 10% of in hospital deaths. [53, 54] However, most patients (up to 75%) are asymptomatic. Traditionally, proximal venous thrombosis are thought to be at highest risk for causing pulmonary emboli; however, the single largest autopsy series ever performed to specifically to look for the source of fatal PE was performed by Havig in 1977, who found that one third of the fatal emboli arose directly from the calf veins. [55]
Superior vena cava syndrome
Superior vena cava syndrome is caused by gradual compression of the superior vena cava (SVC). Patients can present with dyspnea, cough, dysphagia, and swelling of the neck and upper extremities. SVC syndrome is most commonly caused by extrinsic compression from a malignant process, such as lung or breast cancer. However, thrombotic causes of SVC syndrome are increasing due to the more widespread use of central venous catheters and pacemakers. SVC syndrome is a clinical diagnosis, but it can be confirmed with plain radiography, computed tomography (CT) scanning, and venography. [56]
For cancer-related SVC syndrome, the treatment consists of chemotherapy and radiation directed at the obstructing tumor. For thrombotic causes, thrombolysis and anticoagulation may be used. [57] Increasingly, endovascular treatment with balloon dilation and stenting are being used with rapid resolution of symptoms. [58, 59]
For more information, see Superior Vena Cava Syndrome.
Etiology
Numerous factors, often in combination, contribute to deep venous thrombosis (DVT). These may be categorized as acquired (eg, medication, illness) or congenital (eg, anatomic variant, enzyme deficiency, mutation). A useful categorization may be an acute provoking condition versus a chronic condition, as this distinction affects the length of anticoagulant therapy.
The frequent causes of DVT are due to augmentation of venous stasis due to immobilization or central venous obstruction. Immobility can be as transient as that occurring during a transcontinental airplane flight or that during an operation under general anesthesia. Thus, risk factors include obesity, medications, pregnancy, trauma, malignancy, and genetic conditions. [60] It can also be extended, as during hospitalization for pelvic, hip, or spinal surgery, or due to stroke or paraplegia. Individuals in these circumstances warrant surveillance, prophylaxis, and treatment if they develop DVT. [61, 62]
Reduced blood flow from increased blood viscosity or central venous pressure
Increased blood viscosity may decrease venous blood flow. This change may be due to an increase in the cellular component of the blood in polycythemia rubra vera or thrombocytosis or a decrease in the fluid component due to dehydration.
Increased central venous pressure, either mechanical or functional, may reduce the flow in the veins of the leg. Mass effect on the iliac veins or inferior vena cava from neoplasm, pregnancy, stenosis, or congenital anomaly increases outflow resistance.
Anatomic variants contributing to venous stasis
Anatomic variants that result in diminution or absence of the inferior vena cava or iliac veins may contribute to venous stasis. In iliocaval thromboses, an underlying anatomic contributor is identified in 60-80% of patients. The best-known anomaly is compression of left common iliac vein at the anatomic crossing of the right common iliac artery. The vein normally passes under the right common iliac artery during its normal course.
In some individuals, this anatomy results in compression of the left iliac vein and can lead to band or web formation, subsequent stasis, and left leg DVT. The reasons are poorly understood. Compression of the iliac vein is also called May-Thurner syndrome or Cockett syndrome.
Inferior vena cava variants are uncommon. Anomalous development is most commonly detected and diagnosed on cross-sectional imaging or venography. The embryologic evolution of the inferior vena cava is from an enlargement or atrophy of paired supracardinal and subcardinal veins. Anomalous embryologic development may result in absence of the normal cava. These variations may increase the risk of symptoms because small-caliber vessels may be most subject to obstruction. In patients younger than 50 years who have deep venous thrombosis, the incidence of a caval anomaly is as high as 5%. [63]
A double or duplicated inferior vena cava results from lack of atrophy in part of the left supracardinal vein, resulting in a duplicate structure to the left of the aorta. The common form is a partial paired inferior vena cava that connects the left common iliac and left renal veins. When caval interruption, such as placement of a filter, is planned, these alternate pathways must be considered. As an alternative, the inferior vena cava may not develop. The most common alternate route for blood flow is through the azygous vein, which enlarges to compensate. If a venous stenosis is present at the communication of iliac veins and azygous vein, back pressure can result in insufficiency, stasis, or thrombosis. [64]
In rare cases, neither the inferior vena cava nor the azygous vein develops, and the iliac veins drain through internal iliac collaterals to the hemorrhoidal veins and superior mesenteric vein to the portal system of the liver. Hepatic venous drainage to the atrium is patent. Because this pathway involves small hemorrhoidal vessels, thrombosis of these veins can cause severe acute swelling of the legs.
Thrombosis of the inferior vena cava is a rare occurrence and is an unusual result of leg deep venous thrombosis unless an inferior vena cava filter is present and stops a large embolus in the cava, resulting in obstruction and extension of thrombosis. Common causes of caval thrombosis include tumors involving the kidney or liver, tumors invading the inferior vena cava, compression of the inferior vena cava by extrinsic mass, and retroperitoneal fibrosis. [65, 66]
Mechanical injury to vein
Mechanical injury to the vein wall appears to provide an added stimulus for venous thrombosis. Hip arthroplasty patients with the associated femoral vein manipulation represent a high-risk group that cannot be explained by just immobilization, with 57% of thrombi originating in the affected femoral vein rather than the usual site of stasis in the calf. [67] Endothelial injury can convert the normally antithrombogenic endothelium to become prothrombotic by stimulating the production of tissue factor, von Willebrand factor, and fibronectin.
Injury may be obvious, such as those due to trauma, surgical intervention, or iatrogenic injury, but they may also be obscure, such as those due to remote deep venous thrombosis (perhaps asymptomatic) or minor (forgotten) trauma. Previous DVT is a major risk factor for further DVT. The increased incidence of DVT in the setting of acute urinary tract or respiratory infection may be due to an inflammation-induced alteration in endothelial function.
According to the results of a meta-analysis of 64 studies encompassing 29,503 patients, peripherally inserted central catheters (PICCs) may double the risk for DVT in comparison with central venous catheters (CVCs). [68, 69] This was the largest review of the incidence, patterns, and risk for venous thromboembolism (VTE) associated with PICCs yet published; however, the findings were limited by the absence of any published randomized trials.
Compared with CVCs, PICCs were associated with an increased risk of DVT (odds ratio [OR], 2.55; but not of pulmonary embolism (no events). [69] The frequency of PICC-related DVT was highest in patients who were critically ill (13.91%) and patients who had cancer (6.67%).
Common risk factors for deep venous thrombosis
The presence of risk factors plays a prominent role in the assessing the pretest probability of DVT. Furthermore, transient risk factors permit successful short-term anticoagulation, whereas idiopathic deep venous thrombosis or chronic or persistent risk factors warrant long-term therapy.
In the MEDENOX study that evaluated 1102 acutely ill, immobilized admitted general medical patients, multiple logistic regression analysis found the following factors to be significantly and independently associated with an increased risk for VTE, most of which were asymptomatic and diagnosed by venography of both lower extremities [70] :
-
Presence of an acute infectious disease
-
Age older than 75 years
-
Cancer
-
History of prior VTE
The most common risk factors are obesity, previous VTE, malignancy, surgery, and immobility. Each is found in 20-30% of patients. Hospitalized and nursing home patients often have several risk factors and account for one half of all DVT (with an incidence of 1 case per 100 population). [46, 71] Obesity also appears to increases the risk of anticoagulation reversal failure with prothrombin complex concentrate in those with intracranial hemorrhage. [72]
The single most powerful risk marker remains a prior history of DVT, with as many as 25% of acute venous thrombosis occurring in such patients. [73] Pathologically, remnants of previous thrombi are often seen within the specimens of new acute thrombi. However, recurrent thrombosis may actually be the result of primary hypercoagulable states. Abnormalities within the coagulation cascade are the direct result of discrete genetic mutations within the coagulation cascade. Deficiencies of protein C, protein S, or antithrombin III account for approximately 5-10% of all cases of DVT. [74]
Age has been well studied as an independent risk factor for venous thrombosis development. Although a 30-fold increase in incidence is noted from age 30 to age 80, the effect appears to be multifactorial, with more thrombogenic risk factors occurring in the elderly than in those younger than 40 years. [73, 75] Venous stasis, as seen in immobilized patients and paralyzed limbs, also contributes to the development of venous thrombosis. Autopsy studies parallel the duration of bed rest to the incidence of venous thrombosis, with 15% of patients in those studies dying within 7 days of bedrest to greater than 80% in those dying after 12 weeks. [20] Within stroke patients, DVT is found in 53% of paralyzed limbs, compared with only 7% on the nonaffected side. [76]
Malignancy is noted in as many as 30% of patients with venous thrombosis. [73, 77] The thrombogenic mechanisms involve abnormal coagulation, as evidenced by 90% of cancer patients having some abnormal coagulation factors. [78] Chemotherapy may increase the risk of venous thrombosis by affecting the vascular endothelium, coagulation cascades, and tumor cell lysis. The incidence has been shown to increase in those patients undergoing longer courses of therapy for breast cancer, from 4.9% for 12 weeks of treatment to 8.8% for 36 weeks. [79] Additionally, DVT complicates 29% of surgical procedures done for malignancy. [80]
Postoperative venous thrombosis varies depending on a multitude of patient factors, including the type of surgery undertaken. Without prophylaxis, general surgery operations typically have an incidence of DVT around 20%, whereas orthopedic hip surgery can occur in up to 50% of patients. [81] The nature of orthopedic illnesses and diseases, trauma, and surgical repair or replacement of hip and knee joints predisposes patients to the occurrence of VTE disease. These complications are predictable and are the result of alterations of the natural equilibrium mechanisms in various disease states. [82] For more information, see Deep Venous Thrombosis Prophylaxis.
Based on radioactive labeled fibrinogen, about half of lower extremity thrombi develop intraoperatively. [83] Perioperative immobilization, coagulation abnormalities, and venous injury all contribute to the development of surgical venous thrombosis.
Genetic factors
Genetic mutations within the blood’s coagulation cascade represent those at highest risk for the development of venous thrombosis. Genetic thrombophilia is identified in 30% of patients with idiopathic venous thrombosis. Primary deficiencies of coagulation inhibitors antithrombin, protein C, and protein S are associated with 5-10% of all thrombotic events. [84, 85, 86] Altered procoagulant enzyme proteins include factor V, factor VIII, factor IX, factor XI, and prothrombin. Resistance of procoagulant factors to an intact anticoagulation system has also recently been described with the recognition of factor V Leiden mutation, representing 10-65% of patients with DVT. [87] In the setting of venous stasis, these factors are allowed to accumulate in thrombosis prone sites, where mechanical vessel injury has occurred, stimulating the endothelium to become prothrombotic. [88]
Factor V Leiden is a mutation that results in a form of factor Va that resists degradation by activated protein C, leading to a hypercoagulable state. Its importance lies in the 5% prevalence in the American population and its association with a 3-fold to 6-fold increased risk for VTE. Antiphospholipid syndrome is considered a disorder of the immune system, where antiphospholipid antibodies (cardiolipin or lupus anticoagulant antibodies) are associated with a syndrome of hypercoagulability. Although not a normal blood component, the antiphospholipid antibody may be asymptomatic. It is present in 2% of the population, and it may be detected in association with infections or the administration of certain drugs, including antibiotics, cocaine, hydralazine, procainamide, and quinine. [85]
Tests for these genetic defects are often not performed in patients with recurrent venous thrombosis because therapy remains symptomatic. In most patients with these genetic defects, lifetime anticoagulation therapy with warfarin or low molecular weight heparin (LMWH) is recommended after recurrent DVT without an alternative identifiable etiology documented. The risk of recurrent DVT is multiplied 1.4-2 times, with the most common genetic polymorphisms predisposing individuals to DVT. However, the low incidence of factor V Leiden and prothrombin G20210A may not warrant aggressive prophylaxis. Therefore, genetic testing might not be warranted until a second event occurs. [89]
Other conditions that can induce hypercoagulability
Other diseases and states can induce hypercoagulability in patients without other underlying risks for DVT. They can predispose patients to DVT, though their ability to cause DVT without intrinsic hypercoagulability is in question. The conditions include malignancy, dehydration, and use of medications (eg, estrogens). Acute hypercoagulable states also occur, as in disseminated intravascular coagulopathy (DIC) resulting from infection or heparin-induced thrombocytopenia. [90]
Summary of risk factors
A summary of risk factors is as follows:
-
Age
-
Immobilization longer than 3 days
-
Pregnancy and the postpartum period
-
Major surgery in previous 4 weeks
-
Long plane or car trips (>4 hours) in previous 4 weeks
-
Cancer
-
Previous DVT
-
Stroke
-
Acute myocardial infarction (AMI)
-
Congestive heart failure (CHF)
-
Sepsis
-
Nephrotic syndrome
-
Ulcerative colitis
-
Multiple trauma
-
CNS/spinal cord injury
-
Burns
-
Lower extremity fractures
-
Systemic lupus erythematosus (SLE) and the lupus anticoagulant
-
Behçet syndrome
-
Homocystinuria
-
Polycythemia rubra vera
-
Thrombocytosis
-
Inherited disorders of coagulation/fibrinolysis
-
Antithrombin III deficiency
-
Protein C deficiency
-
Protein S deficiency
-
Prothrombin 20210A mutation
-
Factor V Leiden
-
Dysfibrinogenemias and disorders of plasminogen activation
-
Intravenous (IV) drug abuse
-
Oral contraceptives
-
Estrogens
-
Heparin-induced thrombocytopenia (HIT)
Epidemiology
Deep venous thrombosis (DVT) and thromboembolism remain a common cause of morbidity and mortality in bedridden or hospitalized patients, as well as generally healthy individuals. The exact incidence of DVT is unknown because most studies are limited by the inherent inaccuracy of clinical diagnosis. Existing data that probably underestimate the true incidence of DVT suggest that about 80 cases per 100,000 population occur annually. Approximately 1 person in 20 develops a DVT in the course of his or her lifetime. About 600,000 hospitalizations per year occur for DVT in the United States.
In elderly persons, the incidence is increased four-fold. The in-hospital case-fatality rate for venous thromboembolism (VTE) is 12%, rising to 21% in elderly persons. In hospitalized patients, the incidence of venous thrombosis is considerably higher and varies from 20-70%. Venous ulceration and venous insufficiency of the lower leg, which are long-term complications of DVT, affect 0.5% of the entire population. Extrapolation of these data reveals that as many as 5 million people have venous stasis and varying degrees of venous insufficiency.
Age distribution for deep venous thrombosis
Deep venous thrombosis usually affects individuals older than 40 years. The incidence of VTE increases with age in both sexes. The age-standardized incidence of first-time VTE is 1.92 per 1000 person-years.
Prevalence of deep venous thrombosis by sex
The male-to-female ratio is 1.2:1, indicating that males have a higher risk of DVT than females.
Prevalence of deep venous thrombosis by race
From a demographic viewpoint, Asian and Hispanic populations have a lower risk of VTE, whereas whites and blacks have a higher risk (2.5-4 times higher).
Prognosis
Most cases of deep venous thrombosis (DVT) is occult and usually resolves spontaneously without complication. The principal long-term morbidity from DVT is postthrombotic syndrome (PTS), which complicates about a quarter of cases of symptomatic proximal DVT; most cases develop within 2 years afterward.
Death from DVT is attributed to massive pulmonary embolism (PE), which causes as many as 300,000 deaths annually in the United States. [1] PE is the leading cause of preventable in-hospital mortality. The Longitudinal Investigation of Thromboembolism Etiology (LITE) that combined data from two prospective cohort studies, the Atherosclerosis Risk in Communities (ARIC) and the Cardiovascular Health Study (CHS) determined the incidence of symptomatic DVT and pulmonary embolism in 21,680 participants aged 45 years or older who were followed for 7.6 years. [91]
Thromboembolism and recurrent thromboembolism appear to be serious complications of inflammatory bowel disease. [92] In a study comprising 84 patients with inflammatory disease and a history of thromboembolism, of whom, 30% had recurrent thromboembolism, 70 patients (83%) developed venous thromboembolism (40% of which manifested as DVT and 23% as PE). [92]
-
Deep Venous Thrombosis (DVT). This longitudinal ultrasonographic image reveals a partially recanalized thrombus in the femoral vein at the mid thigh.
-
Deep Venous Thrombosis (DVT). Note the principal deep veins of the lower extremity.
-
Deep Venous Thrombosis (DVT). This image demonstrates postphlebitic chronic venous insufficiency with ulceration.
-
Deep Venous Thrombosis (DVT). This image depicts lipodermatosclerosis caused by DVT.
-
Deep Venous Thrombosis (DVT). There is a substantial mortality benefit for fibrinolytic therapy compared to anticoagulation in patients with right ventricular strain from pulmonary embolism (Konstantinides, 1997).
-
Deep Venous Thrombosis (DVT). These sequential images demonstrate treatment of iliofemoral DVT due to May-Thurner (Cockett) syndrome. Far left: View of the entire pelvis reveals iliac occlusion. Middle left: After 12 hours of catheter-directed thrombolysis, an obstruction at the left common iliac vein is evident. Middle right: After 24 hours of thrombolysis, a bandlike obstruction is seen; this is the impression made by the overlying right common iliac artery. Far right: After stent placement, the image shows wide patency and rapid flow through the previously obstructed region. Note that the patient is in the prone position in all views. (Right and left are reversed.)
-
Deep Venous Thrombosis (DVT). This lower-extremity venogram shows outlining of an DVT in the popliteal vein with contrast enhancement.
-
Deep Venous Thrombosis (DVT). The lower-extremity venogram reveals a nonocclusive chronic thrombus. The superficial femoral vein (lateral vein) has the appearance of two parallel veins, when in fact it is one lumen containing a chronic linear thrombus. Although the chronic clot is not obstructive after it recanalizes, it effectively causes the venous valves to adhere in an open position, predisposing the patient to reflux in the involved segment.
-
Deep Venous Thrombosis (DVT). Two views of a commercially available thrombectomy device are shown. The Helix Clot Buster works by creating a vortex with a spinning self-contained propeller that macerates the clot. No thrombolytic agent (ie, tissue plasminogen activator) is necessary when this device is used, but adjunct thrombolytic medications can be useful. Competing devices are available from other manufacturers.
-
Deep Venous Thrombosis (DVT). Popliteal vein thrombosis with normal compression of the common femoral vein is demonstrated. Image courtesy of Very Special Images with permission from Dr Lennard A Nadalo.
-
Deep Venous Thrombosis (DVT). Bilateral popliteal vein thrombosis with normal compression of the superficial femoral vein is shown. Image courtesy of Very Special Images with permission from Dr Lennard A Nadalo.
-
Deep Venous Thrombosis (DVT). Popliteal vein thrombosis is depicted by a Duplex sonogram showing absent flow. Image courtesy of Very Special Images with permission from Dr Lennard A Nadalo.
-
Deep Venous Thrombosis (DVT). Popliteal vein thrombosis is demonstrated by gray-scale images showing compression failure. Image courtesy of Very Special Images with permission from Dr Lennard A Nadalo.
-
Deep Venous Thrombosis (DVT). This contrast-enhanced study was obtained through a Mediport placed through the chest wall through the internal jugular vein to facilitate chemotherapy. A thrombus has propagated peripherally from the tip of the catheter in the superior vena cava into both subclavian veins.
-
Deep Venous Thrombosis (DVT). Superior vena cava syndrome is noted in a patient with lung cancer. The computed tomography scan demonstrates a hypoattenuating thrombus that fills the superior vena cava. The patient was treated with anticoagulation alone.
-
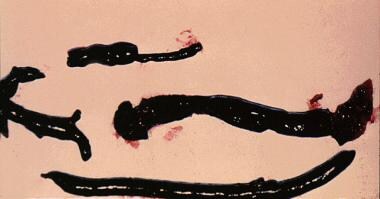
Deep Venous Thrombosis (DVT). The image shows venous thrombi.
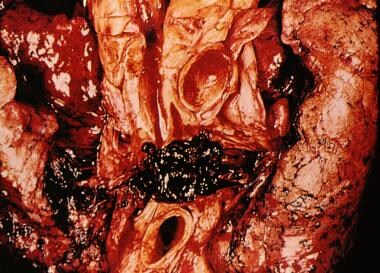
-
Deep Venous Thrombosis (DVT). A pulmonary embolus is shown.
-
Deep Venous Thrombosis (DVT). The coagulation pathway is shown.
-
Deep Venous Thrombosis (DVT). This chart shows postoperative antithrombin III levels.
-
Deep Venous Thrombosis (DVT). This chart depicts perioperative antithrombin III levels and DVT formation.
-
Deep Venous Thrombosis (DVT). Lung scans are shown. LPO = left posterior oblique.
-
Deep Venous Thrombosis (DVT). Spiral computed tomography scan showing a pulmonary thrombus.
-
Deep Venous Thrombosis (DVT). This is the appearance of a normal pulmonary angiogram.
-
Deep Venous Thrombosis (DVT). This is a positive pulmonary angiogram.
-
Deep Venous Thrombosis (DVT). The time course of DVT risk is shown in patients not undergoing treatment for total hip replacement (THR).
-
Deep Venous Thrombosis (DVT). The computed tomography venogram shows bilateral deep venous thrombosis (arrows).
-
Deep Venous Thrombosis (DVT). The high-probability perfusion lung scan shows segmental perfusion defects in the right upper lobe and subsegmental perfusion defects in right lower lobe, left upper lobe, and left lower lobe.
-
Deep Venous Thrombosis (DVT). A normal ventilation scan will make the defects noted in the previous image a mismatch and, hence, a high-probability ventilation-perfusion scan.
-
Deep Venous Thrombosis (DVT). Anterior views of perfusion and ventilation scans are shown. A perfusion defect is present in the left lower lobe, but perfusion to this lobe is intact, making this a high-probability scan.
-
Deep Venous Thrombosis (DVT). A segmental ventilation perfusion mismatch is evident in a left anterior oblique projection.
-
Deep Venous Thrombosis (DVT). This sequence of colored digitized pulmonary angiograms (x-ray) in the front view of the pulmonary arteries in a 43-year-old male patient after a heart attack (cardiac arrest) reveals the presence of a pulmonary embolism with a massive thrombus (clot, dark) in the right and left pulmonary arteries. An outline of the lungs are seen. Image courtesy of Science Source/Zephyr.
-
Deep Venous Thrombosis (DVT). Hematoxylin and eosin stain. This is a low-power view of a thrombus composed of platelets, fibrin, and leukocytes.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- General Principles of Anticoagulation
- Heparin Use in Deep Venous Thrombosis
- Factor Xa and Direct Thrombin Inhibitors
- Duration of Anticoagulation
- Complications of Anticoagulant Therapy
- Emerging Anticoagulant Agents
- Reversal of Anticoagulation
- Pharmacologic Thrombolysis
- General Principles of Endovascular Intervention
- Surgical Thrombectomy
- Placement of Inferior Vena Cava Filters
- Replacement of Venous Valves
- Use of Elastic Compression Stockings
- Ambulation
- Treatment of Superficial Thrombophlebitis
- Treatment of Axillary and Subclavian Vein Thrombosis
- Prophylaxis of Deep Venous Thrombosis
- Show All
- Guidelines
- Medication
- Questions & Answers
- Media Gallery
- Tables
- References