Practice Essentials
Although barbiturates have largely been replaced, both medically and recreationally, by benzodiazepines, barbiturate toxicity still occurs. Clinicians need to be aware not only of the effects of barbiturates alone, but of compound drugs that include barbiturates and barbiturates taken together with alcohol or other synergistic sedatives (see Presentation).
In general, sedative-hypnotic drugs are nonselective in their effects. At lower doses, a reduction in restlessness and emotional tension occurs. At increasingly higher doses, sedation is followed by increasing levels of anesthesia and eventually death.
Toxicity within the barbiturate drug class varies depending on the onset and duration of the agent. For instance, patients with significant poisoning by short-acting barbiturates recover quickly (within 24-48h) in a setting devoid of complications, as opposed to those poisoned with longer-acting agents, such as phenobarbital, which requires more aggressive interventions such as ventilatory support and admission to the ICU.
Treatment for poisoning remains supportive as there is no specific antidote (see Treatment and Medication).
For patient education resources, see Poisoning - First Aid and Emergency Center, Barbiturate Abuse, Drug Overdose, Drug Dependence and Abuse, and Substance Abuse.
Background
Barbiturates are the earliest class of sedative-hypnotic agents to be developed. They were first used in medicine in the early 1900s and remained widely prescribed prior to the development of the less toxic hypnosedative drug class known as benzodiazepines. Their popularity peaked in the 1960s and 1970s for treatment of insomnia, anxiety, and seizures. Also known by street names such as, “reds”, “downers”, “barbs”, “yellow jackets”, “blue heavens”, and “nenbies”, barbiturates’ owe their various effects to a combination of a pyrimidinetrione ring structure and the overall size and structure of the C-5 position substituents.
Barbiturates once enjoyed a central place in the world of recreational drugs at the beginning of the 20th century and were used for a wide range of conditions until their liability for abuse led to an extensive number of barbiturate poisoning cases in the 1950s and 1960s. As late as the turn of the 21st century, barbiturates were commonly used in geriatric suicide involving medication overdose; in a New York City study, 27.2% of fatal overdose suicide cases in elderly persons were due to barbiturates. [1]
Interestingly, as abuse of phenobarbital waned, reported cases of poisoning and abuse from other sedative-hypnotic drugs, such as propofol, ketamine, and gamma-hydroxybutyrate (GHB), steadily increased. The effects that limit the clinical use of these drugs make them appealing to recreational drug users. In a 2007 study, 18% of academic anesthesiology departments surveyed had reported a case of propofol abuse or diversion in the previous 10 years, a 5-fold increase from prior studies; almost all the deaths were in anesthesiology residents. [2]
Despite the decline in barbiturate use, cases of acute poisoning with severe toxicity are still noted at staggering rates in developing countries, where resource limitations and the affordability of barbiturates lend to their increased use as anticonvulsants.
Benzodiazepines have largely replaced barbiturates both medically and recreationally due to their wider therapeutic window, lower drug tolerance, and lower propensity for abuse. Although tolerance to the sedative-hypnotic effects does occur, no tolerance appears to develop to the level at which lethal toxicity occurs. Stricter guidelines dictating barbiturate use have also contributed to its decreased availability.
Procedural sedation and analgesia are essential to ameliorating painful procedures for both adults and children in the emergency department. The favorable pharmacokinetics and adverse effect profile of propofol allows it to be used clinically for procedural sedation. However, propofol contains a relatively narrow therapeutic window and is associated with a dose-dependent risk of bradypnea and hypotension, especially in elderly persons.
While clinically barbiturate use has been largely replaced by benzodiazepines and propofol, there are two distinct scenarios in which their use may still be warranted: status epilepticus and elevated intracranial pressure (ICP).
Being that status epilepticus is the second most frequent neurological emergency and that refractory status epilepticus (RSE) carries a 25% mortality rate, studies indicate that barbiturates still may have a role in this scenario. [3, 4] Supporting this, toxicological or withdraw seizures seem to be more amendable to GABA receptor activation, compared with idiopathic or traumatic seizures, which usually start with a focus of isolated abnormal neurons and are more amendable to blockade of voltage-dependent sodium channels. [5]
In a meta-analysis of the use of barbiturates, propofol, or midazolam in RSE there was no difference in short-term mortality, although immediate effectiveness favored barbiturates. [3] In addition, pentobarbital’s activity at the GABA receptor is less dependent on the presence of adequate normal quantities of GABA, a theoretical benefit in treating of seizures induced by toxins that deplete GABA. Propofol has the advantage of a short half-life which allows for rapid weaning; however, the risks of propofol infusion syndrome needs to be monitored. This has to be weighed against barbiturate long elimination time secondary to its lipophilic nature and high adipose storage (thiopental has a 36-hour elimination half life after continuous infusion). [3]
Although guidelines for management of traumatic brain injury (TBI) recommend that high-dose barbiturate therapy may be considered to lower ICP, [6] the evidence for decreasing morbidity and mortality is lacking. In one study, high-dose barbiturate treatment caused a decrease in ICP in 69% of patients but also caused longer periods of a decreased mean arterial pressure (MAP) despite increased use of high-dose vasopressors. There was no significant effect on outcome. [7] Overall, there is no evidence that barbiturate therapy in patients with TBI improves outcome. [7] This probably is from the fact that cerebral perfusion pressure (CPP) remains unchanged as any benefit in decreasing ICP is offset by a decrease in MAP (CPP=MAP-ICP).
Pathophysiology
Barbiturates bind to specific sites on gamma-aminobutyric acid (GABA)–sensitive ion channels found in the central nervous system (CNS), where they allow an influx of chloride into cell membranes and, subsequently, hyperpolarize the postsynaptic neuron. Although the clinical effects of barbiturates and benzodiazepines are similar and result from hyperpolarization of the neuron, there are subtle differences in terms of receptor binding. Barbiturates increase the duration of Cl ion channel opening at the GABA receptor, which, in turn, increases the efficacy of GABA. Benzodiazepines, on the other hand, increase the frequency of Cl ion channel openings at the GABA receptor, which, in turn, increases the potency of GABA. [8]
GABA and glycine are the major inhibitory neurotransmitters in the CNS. Barbiturates enhance GABA-mediated chloride currents by binding to the GABA. A receptor-ionophore complex at the beta subunit is distinct from the GABA and benzodiazepine binding site and increases the duration of ionophore opening. This potentiates and prolongs the inhibitory actions of GABA. At high doses, barbiturates stimulate GABA A receptors directly in the absence of GABA. Barbiturates also block glutamate (principle excitatory neurotransmitter) receptors (AMPA) in the CNS.
Barbiturates may be grouped functionally into long- and short-acting agents (consisting of ultrashort-, short-, and intermediate-acting agents). However, the relevance of this classification system in terms of prognosis remains to be well defined as the agents’ duration of action is only partially correlated with half-life (the remaining differences are accounted for with tissue binding and distribution). [9] All of the drugs in this class are derivatives of barbituric acid, which was the original compound developed in 1864. However, the structure of each barbiturate differs and can be related to its effective duration of action.
Compared with long-acting agents, short-acting agents are more lipid soluble, more protein bound, have a higher pKa, a more rapid onset, shorter duration of action, and are metabolized almost entirely in the liver to inactive metabolites (which are excreted as glucuronides in the urine). Long-acting agents are less lipid soluble, accumulate more slowly in tissue, and are excreted more readily by the kidney as active drug. For instance, urinary excretion accounts for 20-30% of phenobarbital and 15-42% of primidone elimination (both long-acting agents). Specifically, the duration of action depends mainly on the alkyl groups attached to carbon #5. The structure of these alkyl groups determine lipid solubility of the drug in that the duration of action decreases as the total number of carbons at carbon #5 increases.
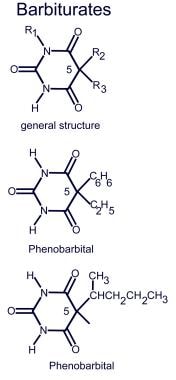
Chemical compounds of barbiturates
See image below.
Short-acting agents have an elimination half-life of less than 40 hours compared with long-acting agents, which have an elimination half-life of longer than 40 hours.
Central nervous system effects
Barbiturates mainly act in the CNS, though they may indirectly affect other organ systems. Direct effects include sedation and hypnosis at lower dosages. The CNS depressant effect mimics that of ethanol. The lipophilic barbiturates, such as thiopental, cause rapid anesthesia because of their tendency to penetrate brain tissue quickly. Elderly people have proportionally more adipose tissue and therefore are more susceptible to this narrow therapeutic index. Barbiturates all have anticonvulsant activity because they hyperpolarize cell membranes. Therefore, they are effective adjuncts in the treatment of epilepsy.
The high doses of barbiturates used in the care of neurocritical patients have in recent years been reported to possibly lead to the accumulation of propylene glycol. Propylene glycol is a commonly used vehicle in the intravenous formulations of many medications, including phenobarbital and pentobarbital. Increased levels of propylene glycol may yield a less recognized complication of therapy, as propylene glycol may exacerbate existing complications associated with large doses of barbiturates, to include hypotension and respiratory depression. In addition, propylene glycol toxicity, ironically, may induce seizures that the barbiturates are intended to treat. [10]
Pulmonary effects
Barbiturates can cause a depression of the medullary respiratory center and induce a respiratory depression. Patients with underlying chronic obstructive pulmonary disease (COPD) are more susceptible to these effects, even at doses that would be considered therapeutic in healthy individuals. Fatality from barbiturate overdose is usually secondary to respiratory depression and subsequent pneumonia and one must respect its narrow therapeutic index as even a slight overdose can cause coma or death.
Cardiovascular effects
Cardiovascular depression may occur following depression of the medullary vasomotor centers; patients with underlying congestive heart failure (CHF) are more susceptible to these effects. At higher doses, cardiac contractility and vascular tone are compromised, which may cause cardiovascular collapse. The combination of the decreased vascular resistance by means of peripheral dilation and inherent negative ionotropic properties of barbiturates yields to the development of another recognized complication, hypotension.
Propofol
Although technically not a barbiturate, the barbiturate-like sedative propofol deserves special mention. It is an ultra–short-acting agent usually used for general anesthesia, procedural sedation, or reduction of intracranial pressure after traumatic brain injury. Propofol binds to GABA A receptors directly and inhibits calcium flow through slow calcium ion channels. [11] Both barbiturates and propofol also interact with N -methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA)/kainite receptors.
Propofol is highly lipid soluble with an onset of less than 1 minute and a quick offset of action. It is barbiturate-like in its activity at the GABA receptor, its pharmacologic effects (respiratory depression and hypotension), and its lipophilic nature. However, its chemical structure is not analogous. Because of its short half-life of 3 minutes, it must be used in an intravenous infusion for long sedation. Additionally, its side effects, particularly respiratory depression, are compounded by benzodiazepines, opioids, and ethanol.
Propofol has specific pharmacokinetics that make it attractive for use in ED procedures. Notably, its rapid onset and short duration of action make it an excellent choice for this purpose. Miner et al compared the efficacy and safety of propofol and etomidate for ED procedures. [12] The success rate was 10% higher in the group given propofol, as 20% of the etomidate group experienced myoclonus. No significant increase in clinical respiratory depression or hypotension occurred in either arm of the study.
Ketamine
Another agent widely used for procedural sedation, and increasingly as a drug of abuse, is ketamine. Ketamine acts primarily on the NMDA receptor by noncompetitive antagonism that decreases the effect of the excitatory neurotransmitter glutamate as it is a derivative of PCP. It also binds to opioid receptors. At low doses (0.1-0.5 mg/kg/h), ketamine induces distortion of time and space, hallucinations, and mild dissociative effects. At larger doses, it induces a more severe dissociation wherein users experience intense detachment, such that their perceptions are completely disconnected from reality. [13] Ketamine causes a sympatheticlike response by inducing bronchodilatation and increasing heart rate and blood pressure. Increased salivation and minimal transient respiratory depression followed by respiratory stimulation may also occur.
GHB interacts with GHB-specific receptors and GABA B receptors. GHB also affects dopamine, opioid, serotonin, acetylcholine, and glutamate neurotransmitter systems. Additional GABA-like effects occur at high doses through the conversion of GHB to GABA. When consumed in oral doses as low as 25 mg/kg, confusion, sedation, respiratory depression, and dizziness have been shown to result. At higher dosages of 50-63 mg/kg, loss of consciousness and profound coma has been documented. [14] Barbiturates stimulate the hepatic cytochrome P-450 mixed function oxidase microsomal enzyme system. Thus, barbiturates affect the drug levels of medications that are dependent on this system and typically increase their metabolism (eg, warfarin [Coumadin]). Note that barbiturates themselves are metabolized by this system, which may partially explain the drug tolerance often observed in long-term users.
Phenibut
While its name is similar to phenobarbital, phenibut (4-amino-3-phenyl-butyric acid) not a barbiturate. Phenibut is a GABA receptor agonist created in the former Soviet Union for the treatment of anxiety, insomnia and alcohol withdrawal with a similar profile to gabapentin and benzodiazepines. It is not approved by the US Food and Drug Administration (FDA) but its use is growing in popularity and is readily available online where it is marketed as a nootropic dietary supplement. [15, 16, 17]
Phenibut's mechanism of action is mainly as a GABA-B receptor agonist and to a lesser extent, GABA-A agonist activity. Symptoms of intoxication including delirium, somnolence, psychosis, and movement disorders. [17] Withdrawal can occur quickly (within 2 hours after last dose), at low doses (2-3g daily) or after a short duration of use (7 days). Symptoms of withdrawal include anxiety, irritability or agitation, insomnia, and psychosis. [15]
Epidemiology
Barbiturate abuse was popular in the 1960s and 1970s. Since then, however, its popularity has waned because of stricter guidelines for use and the introduction of benzodiazepines, which inherently have lower cardiorespiratory toxicity. These two factors have decreased barbiturate availability significantly and have led to less abuse. However, a recent gradual increase in barbiturate abuse has been observed among high school seniors. Street names for phenobarbital include "purple hearts" and "golfballs", while pentobarbital is called "nembies", "yellow jackets", "abbots", or "Mexican yellows".
In 2022, a total of 702 single-substance exposures to barbiturates were reported to US poison control centers. Of these, 33 (4.7%) resulted in major toxicity and 2 deaths were reported. The majority of reported exposures (65%) were in adults; children under the age of 6 years accounted for 19% of exposures. [18]
Since 1965, ketamine has emerged as a common recreational drug among young people and adolescents. Often used in combination with other so-called club drugs such as gamma-hydroxybutyric acid, lysergic acid diethylamide (LSD), and ecstasy, ketamine is commonly called "special K", "kit kat", or "vitamin K".
Propofol abuse has been highlighted by the fatal case of the mega pop star Michael Jackson; however, propofol dependence has been a known problem, especially among anesthesiologists, who have constant access to it. Nearly 1 in 5 of all anesthesiology departments in the United States have reported a case of propofol abuse or diversion. Due to the drug's narrow therapeutic window, at least 7 physician fatalities were reported in 10 years. The trade name for propofol is Diprivan.
Prognosis
With early supportive care, overall in-hospital mortality rates from barbiturate poisoning are less than 0.5-2%.
Fatality associated with barbiturate overdose is rare, but complications are abundant. [18] Morbidity includes immunosuppression with frequent nosocomial infections such as pneumonia, acute respiratory distress syndrome (ARDS), shock, hypoxic damage secondary to prolonged hypotension, and coma. Other complications include iatrogenic ones from forced diuresis, gastric lavage, and central venous access. Life-threatening complications may include acute renal failure, pulmonary edema, and the sequelae of hypotension and respiratory depression. Survivors may develop dermal bullae.
Although propofol is generally considered a safe agent, an entity called propofol infusion syndrome (PRIS) has been recognized, describing acute onset of metabolic acidosis with refractory bradycardia progressing to asystole associated with propofol infusions greater than 48 hours. [19] More than 40 cases have been identified in the literature since 1992 with wide-varied clinical manifestations including rhabdomyolysis, myocardial failure, acute renal failure, cardiac arrest, dyslipidemias, and hypotension.
In a review of 153 case reports published between 1990 and 2014, the more recently published cases describe older patients developing PRIS at lower doses of propofol, in whom arrhythmia, hypertriglyceridaemia and fever are less frequently seen and survival more likely. The propofol infusion rate and duration, the presence of traumatic brain injury and fever were factors independently associated with mortality. Cardiac failure and metabolic acidosis occur early in a dose-dependent manner, while arrhythmia, other electrocardiographic changes and rhabdomyolysis appear more frequently after prolonged propofol infusions, irrespective of dose. Fatality rates decreased over time from 74% before 2001, to 64% between 2001 and 2006, and to 32% in cases reported after 2006. [20]
In addition, multiple cases have been reported in the literature concerning the presentation of ketamine-related bladder dysfunction and lower urinary tract destruction in association with chronic abuse of a new entity of street ketamine. However, no current studies demonstrate a statistically significant difference in urinary system presentations between ketamine and a control group. [11]
-
Chemical compounds of barbiturates.