Introduction and Background
Introduction
Historically, disorders of taste and smell have been difficult to diagnose and treat, often because of a lack of knowledge and understanding of these senses and their disease states. An alteration in taste or smell may be a secondary process in various disease states, or it may be the primary symptom. [1, 2]
In addition to the decreased quality of life as well as the hazards, such as an inability to smell odors such as smoke, natural gas leaks, and spoiled food, associated with loss of smell, there is some evidence of an increase in cognitive decline with smell loss. [3, 4]
The prevalence of disorders of taste and smell in the US general population has been estimated from the US National Health and Nutrition Examination Survey (NHANES) 2011-2014 protocol. A total of 3519 men and women aged 40 and older were tested with a scratch-and-sniff olfactory test; smell, taste, and combined smell and taste impairment had estimated prevalences of 13.5%, 17.3%, and 2.2%, respectively. [5]
It is also known that chemosensory dysfunction deteriorates with age starting in the fifth decade of life. [6] Given the aging of the US population, therefore, it stands to reason that a significant and increasing number of individuals will experience age-related sensory loss. [7] A 2002 study showed that the prevalence of objective olfactory impairment in adults older than 53 years is 24.5% and grows more prevalent with age, reaching 62.5 % in those aged 80-97 years. Extrapolating from these values, there are currently 14 million older adults with some degree of olfactory impairment. Self-reported impairment in this study was only 9.5%, which supports the need for more accurate data based on objective measures. [8]
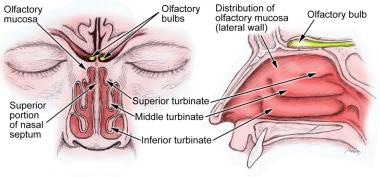
Loss of smell and/or taste has been linked to inadequate nutritional intake, reduced social pleasure, and decreased psychological well-being. It may even be life threatening, impairing the detection of smoke in a fire or the ability to identify spoiled food. Because approximately 80% of taste disorders are truly smell disorders, much of this article focuses on the sense of smell and its dysfunction, with additional discussion of taste and related disorders. See the image below.
Terminology
The disorders of smell are classified as "-osmias" and those of taste as "-geusias."
-
Anosmia - Inability to detect odors [2]
-
Hyposmia - Decreased ability to detect odors
-
Dysosmia - Any smell alteration
Parosmia - Altered perception of smell in the presence of an odor, usually unpleasant
Phantosmia – Perception of smell without an odor present
Agnosia - Inability to classify or contrast odors, although able to detect odors
-
Ageusia - Inability to taste
-
Hypogeusia - Decreased ability to taste
-
Dysgeusia – Distorted ability to taste
Smell and taste disorders can be total (all odors or tastes), partial (affecting several odors or tastes), or specific (only one or a select few odors or tastes).
Anatomy and Physiology
Olfactory system
The olfactory neuroepithelium is located at the upper area of each nasal chamber adjacent to the cribriform plate, superior nasal septum, and superior-lateral nasal wall. It is a specialized pseudostratified neuroepithelium containing the primary olfactory receptors. In neonates, this area is a dense neural sheet, but, in children and adults, the respiratory and olfactory tissues interdigitate. As humans age, the number of olfactory neurons steadily decreases. In addition to the olfactory neurons, the epithelium is composed of supporting cells, Bowman glands and ducts unique to the olfactory epithelium, and basal cells that allow for the regeneration of the epithelium, including the olfactory sensory neurons. [9, 10]
The sense of smell is mediated through stimulation of the olfactory receptor cells by volatile chemicals. To stimulate the olfactory receptors, airborne molecules must pass through the nasal cavity with relatively turbulent air currents and contact the receptors. Odorants can also be perceived by entering the nose posteriorly through the nasopharynx to reach the olfactory receptor via retronasal olfaction. This mechanism is thought to play a key role in the sensation of flavor during eating and drinking. Odorants diffuse into the mucous and are transported to the olfactory receptor. [11] Important determinants of an odor's stimulating effectiveness include duration, volume, and velocity of a sniff.
Each olfactory receptor cell is a primary sensory bipolar neuron. The average nasal cavity contains more than 100 million such neurons. The olfactory neurons are unique because they are generated throughout life by the underlying basal cells. New receptor cells are generated approximately every 30-60 days.
Each regenerating receptor cell extends its axon (CN I) into the CNS as a first-order olfactory neuron and forms synapses with target mitral and tufted cells in the olfactory bulb.
The bipolar olfactory neurons have a short peripheral process and a long central process. The peripheral process extends to the mucosal surface to end in an olfactory knob, which has several immobile cilia forming a dense mat at the mucosal surface. The cilia express the olfactory receptors that interact with odorants. The superfamily of odor receptor proteins are G-protein coupled receptors (GPCRs) associated with adenylate cyclase. The genes that encode them were discovered in 1991 by Linda Buck and Richard Axel, culminating in the Nobel Prize awarded in 2004.
This superfamily encompasses 390 putatively functional genes. It has been found in mice that each neuron expresses only one gene, so odorants are recognized by the complex binding pattern they create. This is most likely the case in humans as well. Interestingly, these genes have been recently discovered to exist in nonolfactory tissues such as sperm and the gut. The function of these genes outside of their role in olfaction is under investigation. [11, 12]
Once an odorant binds to its receptor, a signaling cascade depolarizes the neuron, which sends the signal along its axon, which then converges together within the bundled axons of the fila olfactoria deep to the epithelium.
These axons project through the cribriform plate to the ipsilateral olfactory bulb. The olfactory bulb cells contacted by the olfactory receptor cells include the mitral and tufted cells, arranged in specialized areas termed glomeruli. The axon terminals of receptorlike neurons synapse within the same glomeruli, forming an early topographical odorant map. Therefore, an odor is thought to activate a set of odorant receptors based on its chemical composition. The corresponding glomeruli of the olfactory bulbs are in turn activated, creating a unique pattern of excitation in the olfactory bulb for each odorant.
The glomerular cells are the primary output neurons of the olfactory bulb. Axons from these cells travel to the olfactory cortex, which is divided into 5 parts, including (1) the anterior olfactory nucleus, connecting the 2 olfactory bulbs through the anterior commissure, (2) the olfactory tubercle, (3) the pyriform cortex, which is the main olfactory discrimination region, (4) the cortical nucleus of the amygdala, and (5) the entorhinal area, which projects to the hippocampus.
The olfactory pathway does not involve a thalamic relay prior to its cortical projections. Relays from the olfactory tubercle and the pyriform cortex project to other olfactory cortical regions and to the medial dorsal nucleus of the thalamus and probably involve the conscious perception of odors.
Conversely, the cortical nucleus of the amygdala and the entorhinal area are limbic system components and may be involved in the affective, or hedonic, components of odors. Regional cerebral blood flow (measured with positron emission tomography) is significantly increased in the amygdala with introduction of a highly aversive odorant, and it is associated with subjective ratings of perceived aversiveness.
The vomeronasal organ (VNO), or Jacobson organ, is a bilateral membranous structure located within pits of the anterior nasal septum, deep to the nasal respiratory mucosa and next to the septal perichondria. Its opening in the nasal vestibule is visible in 91-97% of adult humans, and it is 2 cm from the nostril at the junction of the septal cartilage with the bony septum. Unlike lower animals, axons projecting from the VNO have not been found in postnatal humans.
The VNO is believed by some to detect external chemical signals termed pheromones or vomeropherins through neuroendocrine-type cells found within the organ. These signals are not detected as perceptible smells by the olfactory system and may mediate human autonomic, psychologic, and endocrine responses.
Free trigeminal nerve endings, which are stimulated by aversive or pungent stimuli (eg, ammonia, menthol), exist in the nasal mucosa. Odors that stimulate the trigeminal system can induce a sensation of the presence of a strong chemical but without the quality of smell. These stimuli are processed via separate pathways from those in the olfactory system, described above.
Gustatory system
Taste perception is mediated by individual taste buds, with 50-100 tightly packed cells in each bud. Taste buds are made up of modified epithelial cells and chemical receptor cells; however, the receptor cells are not direct neurons as in the olfactory system. Taste bud cells are classified into cell types I-IV and include supporting cells (types I and II), receptor cells (type III), and basal progenitor cells (type IV). [13] These cells have a life span of approximately 10 days and arise continuously from the underlying basal cell layer in a process of constant turnover, similar to that of olfactory receptor neurons. Any bud may contain any of the receptor cells necessary to identify each different taste.
Afferent nerve branches making synaptic contact with receptor cells penetrate the base of the taste bud. Taste buds occupy papillae, which are projections embedded in the tongue epithelium. A single nerve fiber innervates multiple taste papillae, and the nerve contact exerts trophic influences on the epithelium.
The specificity of the gustatory receptor cells is determined by the epithelium in which it resides, not by the particular nerve innervating the bud. A single fiber in the chorda tympani may respond to multiple types of tastes, some tastes more than others. This ability of single nerve fibers to respond to multiple types of stimuli is referred to as broad tuning, and it is shared by the olfactory system.
Lingual papillae have the following four forms, each occupying different areas of the tongue:
-
Fungiform papillae are located in the anterior two thirds of the tongue. People have an average of 33 fungiform papillae with approximately 114 buds per papilla. Innervation is through cranial nerve (CN) VII via the chorda tympani.
-
Circumvallate papillae are located in the posterior two thirds of the tongue, consisting of 8-12 papillae, approximately 250 buds each, for an average of 3000 total buds. Cranial nerve IX innervates these, along with the entire posterior one third of the tongue.
-
Foliate papillae reside in folds and clefts at the lateral borders of the tongue, with approximately 1280 buds. Cranial nerve IX innervates these buds.
-
Filiform papillae have no taste buds.
Other locations of taste buds include the following:
-
Soft palate - Innervated by CN VII via the greater superficial petrosal nerve
-
Epiglottis and larynx - Supplied by the superior laryngeal branch of CN X
-
Pharynx - Supplied by branches from CN IX and CN X
Free trigeminal nerve endings exist on the tongue; these detect strong, often displeasing or irritating sensations in the oral cavity.
Five different taste qualities—salty, sweet, sour, bitter, and umami (monosodium glutamate/5' nucleotide)—have been identified. In addition, there is evidence that another taste perception exists, the ability to sense lipid content. Similar to odorant transmission, most tastes are mediated by various GPCRs. Sweet, umami, and bitter are detected by members of 2 GPCR families, taste 1 receptor family (TAS1R) and taste 2 receptor family (TAS2R). Lipid content is mediated by a free fatty acid receptor. In contrast, sour and salty are mainly mediated by ion channels. [14]
Ongoing research has identified odor and taste receptors in surprising places, including skeletal muscle, brain, respiratory tract, and GI tract. Evidence shows taste receptors present from the stomach to the colon function in nutrient sensing and gut motility. [15, 16] The exact role of these receptors in regions other than the nose and mouth needs to be further elucidated. Research has focused on the presence of bitter taste receptors (TAS2Rs; also known as T2Rs) expressed throughout the body, with differing mechanisms used by these receptors to mediate various nontaste functions; [17] TAS2Rs provide targets for novel therapeutic interventions.
Etiology of Smell and Taste Disorders
Olfactory dysfunction
Disturbances in olfaction can result from pathologic processes at any level along the olfactory pathway. They can be classified in a way analogous to otologic dysfunction, as conductive or sensorineural defects. In conductive (ie, transport) defects, transmission of an odorant stimulus to the olfactory neuroepithelium is disrupted. Sensorineural defects involve the more central neural structures. Overall, the most common causes of primary olfactory deficits are aging, nasal and/or sinus disease, prior viral upper respiratory tract infections (URTIs), and head trauma. [18]
Conductive defects
Inflammatory processes cause a large portion of olfactory defects. These may include rhinitis of various types, including allergic, acute, or toxic (eg, cocaine use). Chronic rhinosinusitis causes progressive mucosal disease and often leads to decreased olfactory function despite aggressive allergic, medical, and surgical intervention.
Masses may block the nasal cavity, preventing the flow of odorants to the olfactory epithelium. These include nasal polyps (most common), inverting papilloma, or any nasal tumor.
Developmental abnormalities (eg, encephaloceles, dermoid cysts) also may cause obstruction.
Patients with laryngectomies or tracheotomies experience hyposmia because of a reduced or absent nasal airflow. Children with tracheotomies who are cannulated very young and for a long period may have a continued problem with olfaction even after decannulation because of a lack of early stimulation of the olfactory system.
Central/sensorineural defects
Infectious and inflammatory processes contribute to central defects in olfaction and in transmission. A viral URTI may result in smell loss by replacing olfactory neuroepithelium with respiratory epithelium, but studies suggest that stem cells remain, allowing for potential regeneration of the olfactory epithelium. Recovery of smell in these cases can take months to years and, in some instances, may never occur. Sarcoidosis (affecting neural structures), Wegener granulomatosis, and multiple sclerosis are also diseases that can result in smell loss. Once thought to be mostly a conductive defect through mucosal edema and polyp formation, chronic rhinosinusitis also appears to disrupt the neuroepithelium with irreversible loss of olfactory receptors through up-regulated apoptosis.
Head trauma, brain surgery, or subarachnoid hemorrhage may stretch, damage, or transect the delicate fila olfactoria or damage brain parenchyma and result in anosmia. [19] A study by Bratt et al found that out of 182 patients with moderate to severe traumatic brain injury (TBI), olfactory dysfunction was diagnosed in 13.7% of them, with 8.2% of the total group suffering from anosmia. The study found an association between olfactory dysfunction and TBI patients who had sustained a fall, skull base fracture, or cortical contusion. [20]
Employing resting-state functional magnetic resonance imaging (rs-fMRI), a study by Park et al found that compared with healthy controls, individuals with traumatic anosmia demonstrated reduced intranetwork connectivity in the olfactory network. However, the olfactory and whole-brain networks had increased internetwork connectivity. Moreover, patients with traumatic anosmia showed decreased modularity and increased global efficiency in the whole-brain network, with these characteristics correlated with disease severity. [21]
A report by Singh et al of 774 TBI admissions found the overall incidence of anosmia to be 19.7%, with the rate of the condition in mild, moderate, and severe TBI being 9.55%, 20.01%, and 43.5%, respectively. [22]
Sense of smell decreases with age, and it has been shown that the number of fibers in the olfactory bulb decreases throughout one's lifetime. In one study the average loss in human mitral cells was 520 cells per year with a reduction in bulb volume of 0.19 mm3. [23] These olfactory bulb losses may be secondary to sensory cell loss in the olfactory mucosa and/or general decline in the regenerative process from stem cells in the subventricular zone.
Congenital syndromes may be associated with neural losses. Kallmann syndrome is one type of congenital smell loss and is due to failed olfactory structure ontogenesis and hypogonadotropic hypogonadism. One study found the vomeronasal organ to be absent in patients with Kallmann syndrome.
Endocrine disturbances (eg, hypothyroidism, hypoadrenalism, diabetes mellitus) may affect olfactory function.
Toxicity of systemic or inhaled drugs (eg, aminoglycosides, formaldehyde) can contribute to olfactory dysfunction. Many other medications and compounds may alter smell sensitivity, including alcohol, nicotine, organic solvents, and direct application of zinc salts. [24]
The NHANES study determined that smoking can be linked to greater risk of olfactory alteration. [25] Research has identified squamous metaplasia and change in the morphology of the olfactory receptor neurons in smokers, as well as a higher level of apoptosis of these neurons in smokers than in controls. In addition, there is evidence that the volume of the olfactory bulb is reduced in smokers. [26]
Various neuropsychiatric disorders (eg depression, [27] seasonal affective disorder, bipolar disorder [28] ) have been linked to hyposmia. It has also been shown that patients with schizophrenia or acute major depressive disorder have not only decreased olfactory sensitivity but also reduced olfactory bulb volumes. [29, 30] The neurologic explanation for these findings is under active investigation and may lead to new therapies or early detection screening tools.
Degenerative processes of the central nervous system (eg, Parkinson disease [PD], Alzheimer disease) and other neurologic diseases (Huntington disease, multiple sclerosis, motor neuron disease) have been associated with hyposmia. Patients with Alzheimer or Parkinson disease show changes in detection, discrimination, and identification of odors compared with age-matched controls. The severity of dysfunction is correlated to disease progression, although in most cases, olfactory loss is present years before motor or cognitive symptoms; this is usually a gradual loss and often goes unnoticed or unreported by patients.
The presence of olfactory dysfunction at the time of PD diagnosis increases the risk of developing dementia. [31] Indeed, in general, olfactory loss in an individual increases the odds of being diagnosed with dementia within 5 years, [32] but this may be a phenomenon of an overall association of age-related sensory impairment with cognitive impairment. [33]
A study by Roos et al, using the University of Pennsylvania Smell Identification Test, the Unified Parkinson’s Disease Rating Scale motor subscale, and the Mini-Mental State Examination, reported a relationship between hyposomia and motor and nonmotor symptoms of PD, including with regard to cognition, depression, anxiety, autonomic dysfunction, and sleep disturbances. It was also linked to the degree to which nigrostriatal dopaminergic cells are lost. In addition, using single-photon emission computed tomography (SPECT) scanning, the investigators found that olfactory test scores were strongly associated with the binding of dopamine transporter in the putamen and caudate nucleus. [34]
A study by Cecchini et al found severe olfactory impairment in persons with Down syndrome, with these individuals performing worse than euploid controls on tests of odor detection threshold, odor discrimination, and odor identification. Among 56 subjects with Down syndrome, 27 demonstrated functional anosmia. [35]
An association has also been recognized between smell loss and increased risk of mortality. [36] This has been found to be unrelated to dementia and may be an indicator of deteriorating health. [37]
The assessment of olfactory function should become a more standard aspect of patient evaluation. There are numerous functional and structural approaches available to assess the olfactory system, including psychosocial and electrophysiological testing, as well as imaging studies. Objective measures of olfactory function may serve as an early marker for these diseases or as a prognostic indicator. An understanding of the mechanism of the decrease in smell could help to further elucidate the pathophysiology of these disorders or uncover new treatments. [38, 39]
Gustatory dysfunction
Much of what is perceived as a taste defect is truly a primary defect in olfaction resulting in an alteration of flavor. The components that comprise the sensation of flavor include the food's smell, taste, texture, and temperature. Each of these sensory modalities is stimulated independently to produce a distinct flavor when food enters the mouth.
Taste may be enhanced by tongue movements, which increase the distribution of the substance over a greater number of taste buds. Adaptation in taste perception exerts a greater influence than in other sensory modalities.
Other than smell dysfunction, the most frequent causes of taste dysfunction are prior URTI, head injury, and idiopathic causes, but many other causes can be responsible.
Lesions at any site from the mucosa, taste buds, unmyelinated nerves, or cranial nerves to the brain stem may impair gustation.
Oral cavity and mucosal disorders including oral infections, inflammation, and radiation-induced mucositis can impair taste sensation. The site of injury with radiotherapy is probably the microvilli of the taste buds, not the taste buds themselves, since taste buds are thought to be radioresistant.
Poor oral hygiene is a leading cause of hypogeusia and cacogeusia. Viral, bacterial, fungal, and parasitic infections may lead to taste disturbances because of secondary taste bud involvement.
Normal aging produces taste loss due to changes in taste cell membranes involving altered function of ion channels and receptors rather than taste bud loss. [7, 40]
A study by Shintani et al found a higher recognition threshold for bitter and salty tastes to be associated with a decreased resting saliva volume. The investigators suggested that “[o]ral moisturization may prevent hypogeusia, particularly in cases of elevated salty or bitter taste thresholds.” [41]
More than 200 medications have been associated with taste disorders. [42] Clinicians need to be aware of this, especially with regard to patients taking numerous drugs.
Malignancies of the head and neck, as well as of other sites, are associated with decreased appetite and inability to appreciate flavors.
Use of dentures or other palatal prostheses may impair sour and bitter perception, and tongue brushing has been shown to decrease taste acuity.
Surgical manipulation may alter taste permanently or temporarily. Resection of the tongue and/or portions of the oral cavity, most commonly for reasons of malignancy, decreases the number of taste buds. Radiation and chemotherapy damage taste receptors and decrease salivary flow, altering taste perception. In otologic surgery, stretching or transection of the chorda tympani nerve may result in temporary dysgeusia. Bilateral injury still may not result in permanent taste dysfunction, because of the alternate innervation through the otic ganglion to the geniculate ganglion via the greater superficial petrosal nerve.
Gastric bypass surgery can also have adverse olfactory and gustatory effects. In a study by Graham et al of 103 patients who underwent Roux-en-Y gastric bypass, sensory changes in taste and smell were reported by 73% and 42% of these individuals, respectively, [43] although patients seem to have less olfactory loss if the bypass is done laparoscopically. [44]
Nutritional deficiencies are involved in taste aberrations. Decreased zinc, copper, and nickel levels can correlate with taste alterations. Nutritional deficiencies may be caused by anorexia, malabsorption, and/or increased urinary losses.
Endocrine disorders also are involved in taste and olfactory disorders. Diabetes mellitus, hypogonadism, Sjögren syndrome, and pseudohypoparathyroidism may decrease taste sensation, while hypothyroidism and adrenal cortical insufficiency may increase taste sensitivity. Hormonal fluctuations in menstruation and pregnancy also influence taste.
AIDS patients often complain of alterations in taste, and detection thresholds of glutamic acid and hydrochloride are higher in patients suffering from AIDS. [45]
Heredity is involved in some aspects of gustation. The ability to taste phenylthiourea (bitter) and other compounds with an –N-C= group is an autosomal dominant trait. Studies have shown that phenylthiourea tasters detect saccharin, potassium chloride (KCl), and caffeine as more bitter. Type I familial dysautonomia (ie, Riley-Day syndrome) causes severe hypogeusia or ageusia because of the absence of taste bud development.
Direct nerve or CNS damage, as in multiple sclerosis, facial paralysis, and thalamic or uncal lesions, can decrease taste perception.
Many other diseases can affect gustation (eg, lichen planus, aglycogeusia, Sjögren syndrome, renal failure with uremia and dialysis, erythema multiforme, geographic tongue, cirrhosis).
COVID-19
Anosmia and dysgeusia are symptoms of coronavirus disease 2019 (COVID-19). [46] The American Academy of Otolaryngology - Head and Neck Surgery (AAO-HNS) recommends that in patients in whom other respiratory diseases, such as allergic rhinitis, acute rhinosinusitis, and chronic rhinosinusitis, are not present, the occurrence of anosmia or hyposmia, as well as dysgeusia, should raise suspicion for COVID-19 infection. [47, 48, 49] The Centers for Disease Control and Prevention (CDC) has added "new loss of taste or smell" to its list of symptoms that may arise 2-14 days after exposure to the COVID-19 virus (ie, severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]). [50] Loss of taste or smell has also been added by the World Health Organization (WHO) to its list of less common COVID-19 symptoms. [51]
A study by Speth et al of 103 patients with COVID-19 found the prevalence of olfactory dysfunction to be 61.2%, with the condition occurring on median infection day 3. A strong correlation was reported between the severity of olfactory dysfunction and the severity of loss of taste. In addition, patients with olfactory dysfunction tended to have more severe shortness of breath. The investigators also found that olfactory dysfunction was less common in older age and more prevalent in females. [52]
Another study, a literature review by Aziz et al, indicated through pooled analysis that taste sensation is altered in almost 50% of patients with COVID-19, although it was suggested that, due to underreporting, the prevalence may be even higher. [53]
A study by Boscolo-Rizzo et al of adult patients with mild COVID-19 reported that within 4 weeks of taste or smell alteration, this symptom partially or completely resolved in 89% of them. [54, 55]
Another study by Boscolo-Rizzo and colleagues found that 92.1% of individuals with baseline smell or taste dysfunction in association with mild COVID-19 reported complete resolution of such dysfunction at 3-year follow-up. Even when smell or taste dysfunction was still present at 2 years, subsequent improvement was possible, with complete or partial resolution in 27.6% and 37.9% of these individuals, respectively, at 3 years. [56]
A European study, by Lechien et al, indicated that loss of smell is much more prevalent in mild cases of COVID-19 (at 85.9%) than in patients with moderate or severe to critical disease (4.5% and 6.9%, respectively). Most patients in the study recovered their sense of smell within 60 days, and almost all subjects did within 6 months. [57, 58]
A study by Renaud et al found that out of 51 patients with COVID-19–related smell loss, 49 were normosmic by 8-month follow-up, with two patients still experiencing hyposmia at 1 year. [59]
A study by Cristillo et al indicated that older age is a risk factor for long-term hyposmia associated with COVID-19. Assessing patients 6 months after they had had mild to moderate COVID-19, the report found that in patients over age 65 years, the likelihood of hyposmia enduring those 6 months was 1.86 times greater than in younger individuals, while for patients over age 75 years, it was 2.67 times greater. In addition, the study found subtle cognitive impairment to be another risk factor for long-term hyposmia in COVID-19. [60]
An Argentinian study also indicated a connection between impairment of smell in COVID-19 and cognitive dysfunction, with preliminary results suggesting that in older adults, the severity of olfactory impairment is a strong predictor of persistent cognitive problems 1 year after infection with SARS-CoV-2. This was in contrast to clinical status, which was not found to significantly predict cognitive dysfunction. [61]
A study by Zazhytska found evidence that in persons with SARS-CoV-2 infection who lose their sense of smell, olfactory receptor genes and key genes in the olfactory receptor signaling pathway are significantly down-regulated. However, the investigators stated that the study does not yet establish whether such down-regulation causes COVID-19–associated anosmia. [62]
A study by Shelton et al indicates that there is a genetic risk factor for loss of taste and smell in COVID-19. The investigators reported that a locus near two genes, UGT2A1 and UGT2A2, is linked to COVID-19–related taste and smell loss. UGT2A1 and UGT2A2, which are expressed in the olfactory epithelium, are involved in odorant metabolization. The study found that the risk factor increases the likelihood of losing taste or smell due to COVID-19 by 11%. [63, 64]
A study by Ho et al indicated that olfactory tissue may suffer axonal injury and microvasculopathy in association with COVID-19. In addition, these were found to be worse in those patients with altered smell. According to the investigators, evidence exists some persons with COVID-19 could experience permanent olfactory system damage and dysfunction. [65]
Diagnosis of Smell and Taste Disorders
The first step in diagnosing any deficit of taste and smell is obtaining a thorough history and physical examination. Give attention to any antecedent URI, nasal or sinus pathology, history of trauma, other medical problems, and medications taken.
Order sinus CT scans if the history and examination are not consistent with a common pattern (eg gradually progressing olfactory loss in a 38-year-old male). Generally, olfactory loss in the absence of CNS symptoms or an abnormal neurologic examination is highly unlikely to be associated with an intracranial mass such as a meningioma. However, an MRI of the brain is often recommended when the history is not straightforward or a secondary neurologic symptom or sign is obtained (eg, 50-year-old woman with a taste phantom that does not resolve after 6 months). Although a standard laboratory panel is not recommended, tests to evaluate for allergy, diabetes mellitus, thyroid functions, renal and liver function, endocrine function, and nutritional deficiencies may be obtained based on history and the physical examination. Olfactory epithelium biopsy is used primarily as a research technique.
Clinical measurement of olfaction
Quantitative measurement of smell and taste dysfunction is most important when chemosensory dysfunction is the primary symptom. The major goal of sensory testing is to assess the degree of chemosensory dysfunction.
Clinical testing can be time consuming and difficult to perform precisely, but some commercially available tests attempt to simplify and standardize these efforts. Often, testing of both nostrils simultaneously is performed to save time; in other sensory systems, however, it is standard to perform unilateral testing to identify pathology, and indeed, olfactory testing of each nostril may be more effective in detecting an olfactory disorder. [66]
Tests of olfactory function that evaluate threshold of odor detection and odor identification have been developed that can provide a reliable measure of olfactory ability. These tests include the butanol threshold test, the University of Pennsylvania Smell Identification Test (UPSIT) (Sensonics, Inc. www.sensonics.com), and the Sniffin' Sticks test (Burghart Messtechnik GmbH www.burghart-mt.de). Measurements of brain electric potentials, ie, olfactory-evoked responses, have been used in research centers along with odor identification tests to evaluate aberrant olfaction with relation to neurologic disease.
Butanol threshold test
The butanol threshold test involves a forced-choice test using an aqueous concentration of butyl alcohol in one sniff bottle and water in the other. The patient is asked to identify the bottle containing the odorant, with each nostril tested separately.
After each incorrect response, the concentration of butanol is increased by a factor of 3 until the patient either achieves 5 correct responses or fails to correctly identify the bottle with 4% butanol.
The detection threshold is recorded as the concentration at which the patient correctly identifies the butanol on 5 consecutive trials. The scoring relates the patient's threshold to a normal subject population
University of Pennsylvania Smell Identification Test
The UPSIT involves 40 microencapsulated odors in a scratch-and-sniff format, with 4 response alternatives accompanying each odor. The patient takes the test alone, with instructions to guess if not able to identify the item.
Anosmic patients tend to score at or near chance (10/40 correct). The scores are compared against sex- and age-related norms, and the results are analyzed. This test has excellent test-retest reliability.
A chart is available relating scores to varying patient populations, including patients with multiple sclerosis, with Korsakoff syndrome, and those feigning anosmia. Those in the latter group tend to score much lower on the test than expected by chance.
Cross-Cultural Smell Identification Test
A variant of the UPSIT, which can be given in 5 minutes, was proposed for a quick measure of olfactory function. The 12-item Cross-Cultural Smell Identification Test (CC-SIT) was developed using input on the familiarity of odors in several countries, including China, Colombia, France, Germany, Italy, Japan, Russia, and Sweden.
The odorants include banana, chocolate, cinnamon, gasoline, lemon, onion, paint thinner, pineapple, rose, soap, smoke, and turpentine. Representatives from each country identified these odorants most consistently.
This test is an excellent alternative for measuring olfactory function in the clinical setting, especially when time is limited, since it is rapid and reliable.
The disadvantage of this test is that its brevity limits its sensitivity in detecting subtle changes in olfactory function.
Sniffin' Sticks
This test, which uses a series of reusable penlike, odor-dispensing devices, evaluates 1) odor threshold through a single staircase method, 2) odor discrimination with forced choice among 3 of 16 different common odorants, and 3) odor identification with multiple forced choice from four verbal items. A composite score is calculated from a composite of all 3 scores to provide an overall assessment of olfactory function.
Olfactory-evoked response (usually reserved for research studies)
To standardize the patient reaction to eye movements, electroencephalogram (EEG) electrodes and an electrooculogram measure olfactory-evoked potentials. A visual tracking task is performed to ensure constant alertness to the task, and headphones playing white noise are worn to mask auditory clues.
Either carbon dioxide (no odor but a trigeminal stimulant) or hydrogen sulfide is delivered via an olfactometer to the nose in a constantly flowing air stream. N1 is the first negative peak measured, and P2 is the second positive trough. Latencies are measured to these two values.
As a common testing method, olfactory-evoked responses are impractical for clinical use. In patients with neurologic disease, the UPSIT revealed abnormality more frequently than did olfactory-evoked responses.
For clinical olfactory function testing, the authors' experience is that the self-administered UPSIT test allows for practical use during a busy clinical practice. However, in the absence of the olfactory tests described above, a simple screening test using a common alcohol pad can be used. The envelope is opened at one end and presented to the patient. With the patient's eyes closed, the pad is then positioned at the level of the umbilicus and slowly brought closer to the nose. The patient is instructed to notify the tester when the alcohol is again detected. The distance of the pad from the nose correlates with the patient's olfactory ability, with a distance of less than 20 cm indicating hyposmia.
Clinical measurement of taste
Evaluation of taste disorders is not as well developed as that of olfaction. It involves measurement of detection or recognition thresholds. No comparable approach to odor identification tests is available because only 5 basic taste sensations exist and only 4 of these (sweet, salty, bitter, and sour) are tested.
Salivary adaptation and size of the tongue area stimulated influence the threshold assessment. Thus, these tests are extremely variable. Changes in threshold detection do not necessarily indicate correlation to changes in suprathreshold taste intensity. Testing of the taste thresholds alone does not provide a full picture of the level of gustatory function or dysfunction. For example, a patient after radiation therapy may recover recognition thresholds for the 4 taste qualities, but the magnitude of the perceived tastes still may be quite depressed.
Threshold detection
Taste Strips (Burghart, Messtechnik, Germany) are strips of paper that can be purchased and are already impregnated with 4 taste qualities: sweet, sour, salty, and bitter, each of these containing 4 different concentrations resulting in 16 strips in total. Specifically, these are sweet (0.4, 0.2, 0.1, 0.05 g/mL sucrose), sour (0.3, 0.165, 0.09, 0.05 g/mL citric acid), salty (0.25, 0.1, 0.04, 0.016 g/mL sodium chloride), and bitter (0.006, 0.0024, 0.0009, 0.0004 g/mL quinine hydrochloride). Normative values for these strips are available.
Magnitude matching
Suprathreshold testing involves assessment of the patient's perceptions of taste intensities at levels above threshold. One method of measuring this quality is with a psychophysical procedure known as magnitude matching.
Other tests of suprathreshold tastes have involved assigning numbers to their sensations, but no direct comparison across individuals can be made. Specific numbers, such as 10 or 100, do not have any intrinsic psychologic value.
Conversely, magnitude matching makes use of one sensory modality that is presumed to be normal (in this case, hearing) in comparison to a deficiency in another sensory modality (taste) by using the following procedure:
Several concentrations of sodium chloride, sucrose, citric acid, and quinine hydrochloric acid, along with several loudness levels of a 1000-Hz tone, are provided for the magnitude matching task. The patient sips each solution and expectorates, and the tones are presented via headphones. The patient provides estimates of perceived magnitude for each stimulus. The results are scaled in relation to loudness functions to reveal abnormalities of taste as depressed psychophysical functions. In other words, patients with hypogeusia associate stronger taste concentrations with weaker tones than normal patients. The major limitations of this testing modality are its dependence on normal hearing and its complicated design, which takes a significant amount of time to administer and analyze.
Spatial test
Taste function in the various areas of the tongue and oral cavity can be measured using a spatial test. Because the gustatory system is multiply innervated, damage to one of the three major nerves (ie, the chorda tympani branch of the facial nerve, the glossopharyngeal nerve, the vagus nerve) or their ganglia may cause a disturbance of taste that can be evaluated only by testing the anatomic areas supplied by those nerves.
To test these areas, 4 standardized sizes of filter paper are soaked with strong concentrations of the 4 basic tastes. The papers are randomly placed on the 4 quadrants of the tongue and on both sides of the soft palate. Patients then identify the quality of the taste and rate its intensity using the same scale as in whole mouth assessment.
Treatment of Olfactory and Gustatory Dysfunction
Treatment of olfactory dysfunction
Any treatment of olfactory disorders must first treat the specific causative abnormality if it has been identified from diagnostic tests, history, and physical examination.
Local nasal and/or sinus conditions should be optimally managed with saline lavage, decongestants, antihistamines, antibiotics, and/or nasal and systemic steroids, if applicable. Polyps and sinus disease that are resistant to medical management should be surgically addressed. Care must be exercised during surgery to avoid damage to the olfactory regions.
Aggressive treatment of these disorders, if present, provides a good chance of improvement. In general, conductive olfactory losses are the most amenable to treatment.
A few of the sensorineural olfactory defects also have specific treatments, but these are fewer and have less chance of success. Generally, viral processes that damage the olfactory neuroepithelium, sarcoidosis, and multiple sclerosis do not have specific remedies (although steroids may be administered in an attempt to limit the inflammation). Indeed, a consensus paper pointed out the lack of existing medical therapies to treat most olfactory disorders, outside of dysfunction arising from inflammatory conditions such as chronic rhinosinusitis and severe allergic rhinitis. [67]
Endocrine disturbances may be addressed by administration of the deficient hormone, as with hypothyroidism. Control of diabetes mellitus may slow neural degeneration of the olfactory system. However, in a study of 12 males with Kallmann syndrome, Gong and Gao stated that, while the condition can be effectively treated with the administration of human chorionic gonadotropin (hCG), human menopausal gonadotropin (hMG), and testosterone undecanoate, there is currently no effective therapy for the olfactory dysfunction occurring in this disease. [68]
Idiopathic cases of olfactory loss are not amenable to specific treatment, although some unproven remedies have been attempted. The best known of these is zinc sulfate. It has not been proven beneficial and is generally regarded as ineffective.
Other unproven remedies include pharmacologic doses of vitamins, topical steroids, and tricyclic antidepressants (for their effect on CSF catecholamines).
It is now known that olfactory training is safe and effective therapy for olfactory losses. [69] This method involves the patient choosing four known odors and intentionally sniffing these odors twice daily. A randomized, controlled study showed improved olfactory function after 18 weeks, particularly in patients who started training within the first 12 months after olfactory dysfunction began. [70] Eliminating toxins (eg, cigarette smoke, airborne pollutants) may help.
A literature review by Sorokowska et al indicated that olfactory training may be an effective treatment in patients with olfactory loss resulting from multiple etiologies, with improvements reported in discrimination between and identification of smells, although not in thresholds of odor detection. [71] However, Jiang et al reported improvements in threshold testing with single-odor olfactory training in patients with traumatic anosmia. [72]
Overall, the patient with olfactory disorders needs reassurance that these generally are not life-threatening problems and that many other individuals experience them. In some patients, psychiatric evaluation and treatment may be warranted. Most importantly, the physician is responsible for warning the patient with olfactory disorders of the hazards associated with the inability to smell odors such as smoke, natural gas leaks, and spoiled food. Smoke detectors, as well as natural gas and propane gas detectors, are commercially available to help eliminate such risks.
Treatment of gustatory dysfunction
As with olfactory problems, direct initial treatment of gustatory dysfunction toward the causative abnormality, if possible.
Address any nasal pathology causing decreased olfaction and thus affecting flavor perception.
Treat mucosal disorders (eg, infections, inflammations). Treat oral candidiasis and other local factors, and replete any vitamin deficiency that may cause glossitis.
Aid patients in eliminating local irritants (eg, mouthwashes, ill-fitting dentures)
In mucositis or dry mouth as a result of radiation therapy, artificial saliva or salivary stimulants and local anti-inflammatory medications may improve some taste dysfunction.
Correcting endocrine disorders with the appropriate hormone replacement may improve the taste disorder.
Consider eliminating a medication suspected of causing dysgeusia unless the medication is crucial in treating another medical problem and cannot be substituted.
In the case of familial dysautonomia, in which patients have a complete lack of lingual taste buds, subcutaneous administration of methacholine has been reported to normalize previously elevated taste thresholds for all taste qualities. The cholinergic mechanism is probably related to taste transduction via free nerve endings because these patients have no taste receptors.
Some gustatory deficits are untreatable (eg, some cases of nerve or CNS damage, end-stage diabetic neuropathy, multiple sclerosis).
Advise patients that chewing food well increases the release of the tastant and increases saliva production to further distribute the chemicals. Switching foods during the meal decreases the phenomenon of adaptation and can improve detection of the tastes.
Finally, for patients who are anosmic or hyposmic (including many elderly people), simulated odors are available to use while cooking to augment the sensation of flavor. A drawback of these simulated odors is that, to normosmic people, the smell is quite pungent. Thus, these odors cannot be used in mixed groups of anosmic and normosmic individuals.
Summary
Smell and taste disorders traditionally have been overlooked in most aspects of medical practice because these specialized senses often are not considered critical to life. However, they affect everyday enjoyment of food, and they impair detection of the potentially dangerous smells of smoke or spoiled food.
Anxiety and depression, as well as anorexia and nutritional deficiencies, may result from taste and smell disorders. In the aforementioned study by Singh et al, the investigators reported that at 1-year follow-up, 60% of TBI patients with anosmia suffered from depression, versus 36% of those without anosmia. [22]
Many causes of smell and taste disorders exist, and the modalities of treatment begin with treating the specific deficit, if possible. Unfortunately, much about the diagnosis and treatment of taste and smell dysfunction remains to be discovered. Most "taste" defects are truly alterations in perception of flavor due to smell defects, and they should be treated accordingly.
Some standardized tests, such as the butanol threshold, odor identification, Sniffin' Sticks, UPSIT, and olfactory-evoked potentials, can help diagnose and measure olfactory dysfunction.
Reassurance is one of the most important aspects of treatment in these disorders because cures are often difficult to obtain and spontaneous resolution may take weeks, months, or years.
Questions & Answers
Overview
Why are taste and smell disorders difficult to diagnose?
What is the prevalence of taste and smell disorders?
What is the morbidity associated with taste and smell disorders?
How are taste and smell disorders classified?
What is the anatomy of the olfactory system relevant to taste and smell disorders?
What is the role of the olfactory system in the pathophysiology of taste and smell disorders?
What does the development of taste and smell disorders in the olfactory system involve?
What is the role of the gustatory system in the pathophysiology of taste and smell disorders?
What is the anatomy of lingual papillae relevant to taste and smell disorders?
Where are extralingual taste buds located?
What is the pathophysiology of taste perception in taste disorders?
What is the role of receptors throughout the body in the pathophysiology taste and smell disorders?
What causes olfactory dysfunction?
What is the role of conductive defects in the etiology of taste and smell disorders?
What is the role of infection in the etiology of taste and smell disorders?
What is the role of brain injury in the etiology of taste and smell disorders?
What is the role of normal aging in the etiology of taste and smell disorders?
Which congenital disorders are associated with taste and smell disorders?
Which endocrine disorders are associated with taste and smell disorders?
What is the role of drugs in the etiology of taste and smell disorders?
What is the role of smoking in the etiology of taste and smell disorders?
Which psychiatric disorders are associated with taste and smell disorders?
Which neurologic disorders are associated with taste and smell disorders?
What is the role of taste and smell disorders in Down syndrome?
How do taste and smell disorders affect mortality risk?
Why should assessment of olfactory function be included in standard patient evaluation?
How are taste and smell disorders diagnosed?
What is the role of sensory testing in the diagnosis of taste and smell disorders?
What is the role of butanol threshold test in the diagnosis of taste and smell disorders?
What is the role of Sniffin' Sticks in the diagnosis of taste and smell disorders?
What is the role of olfactory-evoked response in the diagnosis of taste and smell disorders?
What is included in the evaluation of taste disorders?
What is the role of taste strips in the diagnosis of taste and smell disorders?
What is the role of magnitude matching in the diagnosis of taste and smell disorders?
What is the role of spatial testing in the diagnosis of taste and smell disorders?
How is olfactory dysfunction in taste and smell disorders treated?
How is gustatory dysfunction in taste and smell disorders treated?
What are taste and smell disorders?
-
Head anatomy with olfactory nerve.
-
Olfactory receptors.