Practice Essentials
Inflammatory bowel disease (IBD) is an idiopathic disease caused by a dysregulated immune response to host intestinal microflora. The two major types of inflammatory bowel disease are ulcerative colitis (UC), which is limited to the colonic mucosa, and Crohn disease (CD), which can affect any segment of the gastrointestinal tract from the mouth to the anus, involves "skip lesions," and is transmural. There is a genetic predisposition for IBD, and patients with this condition are more prone to the development of malignancy. See the image below.
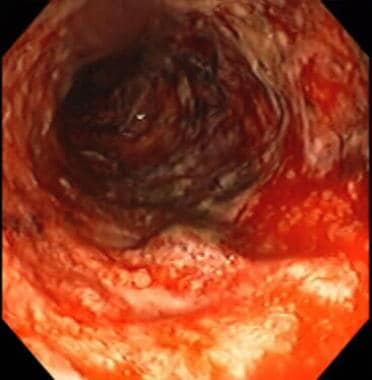 Inflammatory bowel disease. Severe colitis noted during colonoscopy in a patient with inflammatory bowel disease. The mucosa is grossly denuded, with active bleeding noted. The patient had her colon resected very shortly after this view was obtained.
Inflammatory bowel disease. Severe colitis noted during colonoscopy in a patient with inflammatory bowel disease. The mucosa is grossly denuded, with active bleeding noted. The patient had her colon resected very shortly after this view was obtained.
Signs and symptoms
Generally, the manifestations of IBD depend on the area of the intestinal tract involved. The symptoms, however, are not specific for this disease. They are as follows:
-
Abdominal cramping
-
Irregular bowel habits, passage of mucus without blood or pus
-
Weight loss
-
Fever, sweats
-
Malaise, fatigue
-
Arthralgias
-
Growth retardation and delayed or failed sexual maturation in children
-
Extraintestinal manifestations (10%-20%): Arthritis, uveitis, or liver disease
-
Grossly bloody stools, occasionally with tenesmus: Typical of UC, less common in CD
-
Perianal disease (eg, fistulas, abscesses): Fifty percent of patients with CD
The World Gastroenterology Organization indicates the following symptoms may be associated with inflammatory damage of the digestive tract [1] :
-
Diarrhea: Possible presence of mucus/blood in stool; occurs at night; incontinence
-
Constipation: May be the primary symptom in UC limited to the rectum; obstipation may occur; may proceed to bowel obstruction
-
Bowel movement abnormalities: Possible presence of pain or rectal bleeding, severe urgency, tenesmus
-
Abdominal cramping and pain: Commonly present in the right lower quadrant in CD; occur in the periumbilical or in the left lower quadrant in moderate to severe UC
-
Nausea and vomiting: More often in CD than in UC
See Presentation for more detail.
Diagnosis
Examination in patients with IBD may include the following findings, which are directly related to the severity of the attack:
-
Fever
-
Tachycardia
-
Dehydration
-
Toxicity
-
Pallor, anemia
-
Toxic megacolon: Medical emergency; patients appear septic, have high fever, lethargy, chills, and tachycardia, as well as have increasing abdominal pain, tenderness, distention
-
Mass in the right lower abdominal quadrant: May be present in CD
-
Perianal complications: May be observed in up to 90% of cases of CD [2]
Testing
Although several laboratory studies may aid in the management of IBD and provide supporting information, no laboratory test is specific enough to adequately and definitively establish the diagnosis, including the following:
-
Complete blood count
-
Nutritional evaluation: Vitamin B12 evaluation, iron studies, red blood cell folate, nutritional markers
-
Erythrocyte sedimentation rate and C-reactive protein levels
-
Fecal calprotectin level
-
Serologic studies: Perinuclear antineutrophil cytoplasmic antibodies (ANCA), anti-Saccharomyces cerevisiae antibodies (ASCA)
-
Stool studies: Stool culture, ova and parasite studies, bacterial pathogens culture, and evaluation for Clostridium difficile infection [3]
Imaging studies
The following imaging studies may be used to assess patients with IBD:
-
Upright chest and abdominal radiography
-
Barium double-contrast enema radiographic studies
-
Abdominal ultrasonography
-
Abdominal/pelvic computed tomography scanning/magnetic resonance imaging
-
Computed tomography enterography
-
Colonoscopy, with biopsies of tissue/lesions
-
Flexible sigmoidoscopy
-
Upper gastrointestinal endoscopy
-
Capsule enteroscopy/double balloon enteroscopy
See Workup for more detail.
Management
The medical approach for patients with IBD is symptomatic care (ie, relief of symptoms) and mucosal healing for mild disease (eg, erythema without ulceration) following a stepwise approach to medication, with escalation of the medical regimen until a response is achieved (“step-up” or “stepwise” approach) for mild disease (eg, erythema without ulceration), such as the following:
-
Step I – Aminosalicylates (oral, enema, suppository formulations): For treating flares and maintaining remission; more effective in UC than in CD
-
Step IA – Antibiotics: Used sparingly in UC (limited efficacy, increased risk for antibiotic-associated pseudomembranous colitis); in CD, most commonly used for perianal disease, fistulas, intra-abdominal inflammatory masses
-
Step II – Corticosteroids (intravenous, oral, topical, rectal): For acute disease flares only
-
Step III – Immunomodulators and biologics: Effective for steroid-sparing action in refractory disease; primary treatment for fistulas and maintenance of remission in patients intolerant of or not responsive to aminosalicylates
-
Step IV – Clinical trial agents: Tend to be disease-specific (ie, an agent works for CD but not for UC, or vice versa)
Moderate to severe disease warrants more aggressive initial treatment (eg, immunomodulators or biologic agents) in order to prevent complications of the disease.
Pharmacotherapy
The following medications may be used in patients with IBD:
-
5-Aminosalicylic acid derivatives (eg, sulfasalazine, mesalamine, balsalazide, olsalazine)
-
Antibiotics (eg, metronidazole, ciprofloxacin, rifaximin)
-
Corticosteroid agents (eg, hydrocortisone, prednisone, methylprednisolone, prednisolone, budesonide, dexamethasone)
-
Immunosuppressant agents (eg, azathioprine, 6-mercaptopurine, methotrexate, cyclosporine, tofacitinib)
-
Biologic agents, including tumor necrosis factor (TNF) inhibitors (eg, infliximab, adalimumab, certolizumab pegol); anti-integrin antibodies (eg, natalizumab); inhibitors of IL-12 and IL-23 (eg, ustekinumab)
-
H2-receptor antagonists (eg, cimetidine, ranitidine, famotidine, nizatidine)
-
Proton pump inhibitors (eg, omeprazole, lansoprazole, esomeprazole magnesium, rabeprazole sodium, pantoprazole)
-
Antidiarrheal agents (eg, diphenoxylate and atropine, loperamide, cholestyramine)
-
Anticholinergic antispasmodic agents (eg, dicyclomine, hyoscyamine)
Surgery
UC is surgically curable. However, surgical resection is not curative in CD, with recurrence being the norm. Consider early consultation with a surgeon in the setting of severe colitis or bowel obstruction and in cases of suspected toxic megacolon.
Surgical intervention in IBD includes the following:
-
UC: Proctocolectomy with ileostomy, total proctocolectomy with ileoanal anastomosis
-
Fulminant colitis: Surgical procedure of choice is subtotal colectomy with end ileostomy and creation of a Hartmann pouch
-
CD: Surgery (not curative) most commonly performed in patients with complications of the disease; generally consists of conservative resection (eg, potential stricturoplasty vs resective surgery) to preserve bowel length in case future additional surgery is needed [4]
-
Selected patients with distal ileal or proximal colonic disease: Option for ileorectal or ileocolonic anastomosis
-
Severe perianal fistulas: Option for diverting ileostomy; generally, resection for symptomatic enteroenteric fistulas
See Treatment and Medication for more detail.
Background
Inflammatory bowel disease (IBD) is an idiopathic disease caused by a dysregulated immune response to host intestinal microflora. The two major types of IBD are ulcerative colitis (UC), which is limited to the colon, and Crohn disease (CD), which can involve any segment of the gastrointestinal (GI) tract from the mouth to the anus, involves "skip lesions," and is transmural (see the images below). There is a genetic predisposition for IBD (see Etiology), and patients with this condition are more prone to the development of malignancy (see Prognosis).
 Inflammatory bowel disease. Severe colitis noted during colonoscopy in a patient with inflammatory bowel disease. The mucosa is grossly denuded, with active bleeding noted. The patient had her colon resected very shortly after this view was obtained.
Inflammatory bowel disease. Severe colitis noted during colonoscopy in a patient with inflammatory bowel disease. The mucosa is grossly denuded, with active bleeding noted. The patient had her colon resected very shortly after this view was obtained.
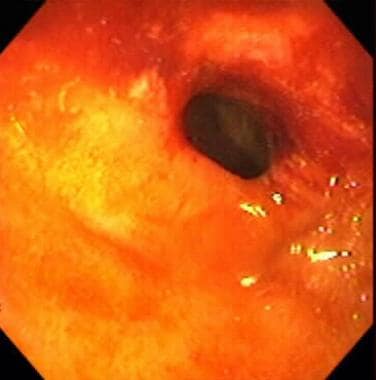 Inflammatory bowel disease. Stricture in the terminal ileum noted during colonoscopy in a patient with inflammatory bowel disease. This image depicts a narrowed segment visible upon intubation of the terminal ileum with the colonoscope. Relatively little active inflammation is present, indicating that this is a cicatrix stricture.
Inflammatory bowel disease. Stricture in the terminal ileum noted during colonoscopy in a patient with inflammatory bowel disease. This image depicts a narrowed segment visible upon intubation of the terminal ileum with the colonoscope. Relatively little active inflammation is present, indicating that this is a cicatrix stricture.
Ulcerative colitis and Crohn disease share many extraintestinal manifestations, although some of these tend to occur more commonly with either condition (see the image below). Eye-skin-mouth-joint extraintestinal manifestations (eg, oral aphthae, erythema nodosum, large-joint arthritis, and episcleritis) reflect active disease, whereas pyoderma gangrenosum, primary sclerosing cholangitis (PSC), ankylosing spondylitis, uveitis, kidney stones, and gallstones may occur in quiescent disease. [5]
Although both ulcerative colitis and Crohn disease have distinct pathologic findings, approximately 10%-15% of patients cannot be classified definitively into either type; in such patients, the disease is labeled as indeterminate colitis. Systemic symptoms are common in IBD and include fever, sweats, malaise, and arthralgias.
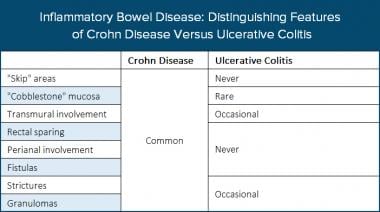 Inflammatory bowel disease. The table distinguishes features of Crohn disease (CD) and ulcerative colitis (UC).
Inflammatory bowel disease. The table distinguishes features of Crohn disease (CD) and ulcerative colitis (UC).
The rectum is always involved in ulcerative colitis, and the disease primarily involves continuous lesions of the mucosa and the submucosa. Both ulcerative colitis and Crohn disease usually have waxing and waning intensity and severity. When the patient is symptomatic due to active inflammation, the disease is considered to be in an active stage (the patient is having a flare of the IBD). (See Presentation.)
In many cases, symptoms correspond well to the degree of inflammation present for either disease, although this is not universally true. In some patients, objective evidence linking active disease to ongoing inflammation should be sought before administering medications with significant adverse effects (see Medication), because patients with IBD can have other reasons for their gastrointestinal symptoms unrelated to their IBD, including coexisting irritable bowel syndrome (IBS), celiac disease, or other confounding diagnoses, such as nonsteroidal anti-inflammatory drug (NSAID) effects and ischemic or infectious colitis.
Although ulcerative colitis and Crohn disease have significant differences, many, but not all, of the treatments available for one condition are also effective for the other. Surgical intervention for ulcerative colitis is curative for colonic disease and potential colonic malignancy, but it is not curative for Crohn disease. (See Treatment.)
Pathophysiology
The common end pathway of ulcerative colitis is inflammation of the mucosa of the intestinal tract, causing ulceration, edema, bleeding, and fluid and electrolyte loss. [6] In several studies, genetic factors appeared to influence the risk of inflammatory bowel disease (IBD) by causing a disruption of epithelial barrier integrity, deficits in autophagy, [7] deficiencies in innate pattern recognition receptors, and problems with lymphocyte differentiation, especially in Crohn disease. [8]
Inflammatory mediators have been identified in IBD, and considerable evidence suggests that these mediators play an important role in the pathologic and clinical characteristics of these disorders. Cytokines, which are released by macrophages in response to various antigenic stimuli, bind to different receptors and produce autocrine, paracrine, and endocrine effects. Cytokines differentiate lymphocytes into different types of T cells. Helper T cells, type 1 (Th-1), are associated principally with Crohn disease, whereas Th-2 cells are associated principally with ulcerative colitis. The immune response disrupts the intestinal mucosa and leads to a chronic inflammatory process. [9]
In animal studies, a local irritant (eg, acetic acid, trinitrobenzene sulfonic acid) can be inserted via an enema into the colon of rats or rabbits to induce a chemical colitis. An interleukin-10 (IL-10) knockout mouse has been genetically engineered to have some characteristics similar to those of a human with IBD. The cotton-top marmoset, a South American primate, develops colitis very similar to ulcerative colitis when the animal is subjected to stress.
Ulcerative colitis
In ulcerative colitis, the inflammation begins in the rectum and extends proximally in an uninterrupted fashion to the proximal colon and could eventually involve the entire length of the large intestine. The rectum is always involved in ulcerative colitis; and unlike in Crohn disease, there are no "skip areas" (ie, normal areas of the bowel interspersed with diseased areas), unless pretreated with topical rectal therapy (ie, a steroid or 5-aminosalicylic acid [5-ASA] enema).
The disease remains confined to the rectum in approximately 25% of cases, and in the remainder of cases, ulcerative colitis spreads proximally and contiguously. Pancolitis occurs in 10% of patients. The distal terminal ileum may become inflamed in a superficial manner, referred to as backwash ileitis. Even with less than total colonic involvement, the disease is strikingly and uniformly continuous. As ulcerative colitis becomes chronic, the colon becomes a rigid foreshortened tube that lacks its usual haustral markings, leading to the lead-pipe appearance observed on barium enema. (See the images below.)
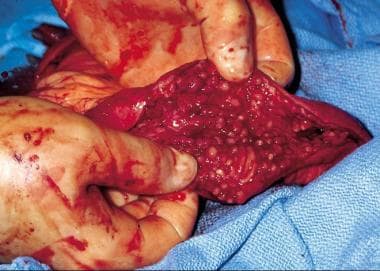 Inflammatory bowel disease. Inflamed colonic mucosa demonstrating pseudopolyps in a patient with ulcerative colitis.
Inflammatory bowel disease. Inflamed colonic mucosa demonstrating pseudopolyps in a patient with ulcerative colitis.
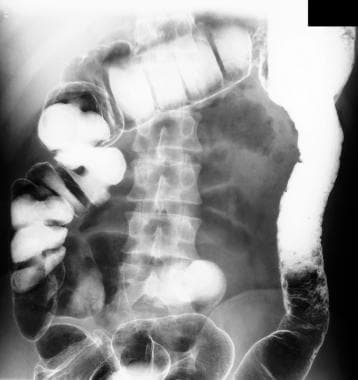 Inflammatory bowel disease. Double-contrast barium enema study shows pseudopolyposis of the descending colon in a patient with ulcerative colitis.
Inflammatory bowel disease. Double-contrast barium enema study shows pseudopolyposis of the descending colon in a patient with ulcerative colitis.
Crohn disease
Crohn disease can affect any portion of the gastrointestinal tract, from the mouth to the anus, and causes three patterns of involvement: inflammatory disease, strictures, and fistulas. This disease consists of segmental involvement by a nonspecific granulomatous inflammatory process. The most important pathologic feature of Crohn disease is that it is transmural, involving all layers of the bowel, not just the mucosa and the submucosa, which is characteristic of ulcerative colitis. Furthermore, Crohn disease is discontinuous, with skip areas interspersed between two or more involved areas.
Late in the disease, the mucosa develops a cobblestone appearance, which results from deep, longitudinal ulcerations interlaced with intervening normal mucosa (see the images below). In 35% of cases, Crohn disease occurs in the ileum and colon; in 32%, solely in the colon; in 28%, in the small bowel; and in 5%, in the gastroduodenal region. [10] Diarrhea, cramping, and abdominal pain are common symptoms of Crohn disease in all of the above locations, except for the gastroduodenal region, in which anorexia, nausea, and vomiting are more common. [10]
Rectal sparing is a typical but not constant feature of Crohn disease. However, anorectal complications (eg, fistulas, abscesses) are common. Much less commonly, Crohn disease involves the more proximal parts of the GI tract, including the mouth, tongue, esophagus, stomach, and duodenum.
 Inflammatory bowel disease. Cobblestone change of the mucosa of the terminal ileum in a patient with Crohn disease. Communicating fissures and crevices in the mucosa separate islands of more intact, edematous epithelium.
Inflammatory bowel disease. Cobblestone change of the mucosa of the terminal ileum in a patient with Crohn disease. Communicating fissures and crevices in the mucosa separate islands of more intact, edematous epithelium.
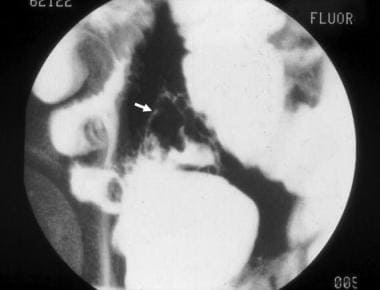 Inflammatory bowel disease. This computed tomography scan from a patient with terminal ileal Crohn disease shows an enteroenteral fistula (arrow) between loops of diseased small intestine.
Inflammatory bowel disease. This computed tomography scan from a patient with terminal ileal Crohn disease shows an enteroenteral fistula (arrow) between loops of diseased small intestine.
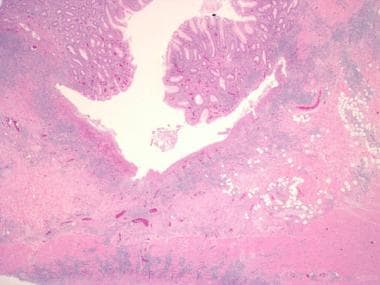 Inflammatory bowel disease. Another example of a deep, fissuring ulcer in a patient with Crohn disease. Note the increase in submucosal inflammation and scattered lymphoid aggregates.
Inflammatory bowel disease. Another example of a deep, fissuring ulcer in a patient with Crohn disease. Note the increase in submucosal inflammation and scattered lymphoid aggregates.
Cholelithiasis and nephrolithiasis
The incidence of gallstones and kidney stones is increased in Crohn disease because of malabsorption of fat and bile salts. Gallstones are formed because of increased cholesterol concentration in the bile, which is caused by a reduced bile salt pool.
Patients who have Crohn disease with ileal disease or ileal resection are also likely to form calcium oxalate kidney stones. With the fat malabsorption, unabsorbed long-chain fatty acids bind calcium in the lumen. Oxalate in the lumen is normally bound to calcium. Calcium oxalate is poorly soluble and poorly absorbed; however, if calcium is bound to malabsorbed fatty acids, oxalate combines with sodium to form sodium oxalate, which is soluble and is absorbed in the colon (enteric hyperoxaluria). The development of calcium oxalate stones in Crohn disease requires an intact colon to absorb oxalate. Patients with ileostomies generally do not develop calcium oxalate stones, but they may develop uric acid or mixed stones. [11]
Etiology
Three characteristics define the etiology of inflammatory bowel disease (IBD): (1) genetic predisposition; (2) an altered, dysregulated immune response; and (3) an altered response to gut microorganisms. However, the triggering event for the activation of the immune response in IBD has yet to be identified. Possible factors related to this event include a pathogenic organism (as yet unidentified) or an inappropriate response (ie, failure to downgrade the inflammatory response to an antigen, such as an alteration in barrier function).
No mechanism has been implicated as the primary cause, but many are postulated. The lymphocyte population in persons with IBD is polyclonal, making the search for a single precipitating cause difficult. In any case, an inappropriate activation of the immune system leads to continued inflammation of the intestinal tract, with both an acute (neutrophilic) and chronic (lymphocytic, histiocytic) inflammatory response.
Several environmental risk factors have been proposed as contributing to the IBD pathogenesis, but the results are inconsistent, and the limitations of the studies preclude drawing firm conclusions. The most consistent association described has been smoking, which increases the risk of Crohn disease. However, current smoking protects against ulcerative colitis, whereas former smoking increases the risk of ulcerative colitis. Dietary factors have also been inconsistently described. In some studies, high fiber intake and high intake of fruits and vegetables appear protective against IBD. [12] The E3N prospective study found that high animal protein intake (meat or fish) carried a higher risk of IBD. [13]
Genetics
Persons with IBD have a genetic susceptibility for the disease, [14] and considerable research over the past decade has improved our understanding of the role of these genes. Note that these genes appear to be permissive (ie, allow IBD to occur), but they are not causative (ie, just because the gene is present does not necessarily mean the disease will develop).
First-degree relatives have a 5- to 20-fold increased risk of developing IBD, as compared with persons from unaffected families. [6, 8] The child of a parent with IBD has a 5% risk of developing IBD. Twin studies show a concordance of approximately 70% in identical twins, versus 5%-10% in nonidentical twins. Of patients with IBD, 10%-25% are estimated to have a first-degree relative with the disease. Monozygous twin studies show a high concordance for Crohn disease but less so for ulcerative colitis.
Crohn disease
An early discovery on chromosome 16 (IBD1 gene) led to the identification of 3 single nucleotide polymorphisms (2 missense, 1 frameshift) in the NOD2 gene (now called CARD15) as the first gene (CARD15) clearly associated with IBD (as a susceptibility gene for Crohn disease). CARD15 is a polymorphic gene involved in the innate immune system. The gene has more than 60 variations, of which 3 play a role in 27% of patients with Crohn disease, primarily in patients with ileal disease.
Subsequent studies have suggested that the CARD15 genotype is associated not only with the onset of the disease but also with its natural history. A study on a German and Norwegian cohort showed that patients with 1 of the 3 identified risk alleles for CARD15 were more likely to have either ileal or right colonic disease. [15, 16] These gene products appear to be involved in the intracellular innate immune pathways that recognize microbial products in the cytoplasm.
Another early genome-wide association study looked at Jewish and non-Jewish case-control cohorts and identified 2 single nucleotide polymorphisms in the IL23R gene, which encodes 1 subunit of the interleukin-23 receptor protein. [17] Interestingly, this study also described the promising nature of certain therapies that block the function of IL-23. Further research suggested that one particular polymorphism in the IL23R gene showed the strongest association in a German population. [18] However, another study found that the Arg381Gln substitution is associated with childhood onset of IBD in Scotland. [19] These gene products appear to be involved in regulating adaptive immunity.
Numerous other loci have been identified as conferring susceptibility to Crohn disease, including several large meta-analyses that found multiple novel susceptibility loci and confirmed earlier findings. In one meta-analysis of 3 genome-wide association scans, 526 single nucleotide polymorphisms from 74 distinct genomic loci were found. [20] In sorting out loci that have been previously discussed, there were 21 new loci that were associated with an increased risk of developing Crohn Disease and have functional implications, including the genes CCR6, IL12B, STAT3, JAK2, LRRK2, CDKAL1, and PTPN22. [20] Most of these genes are involved in signal transduction in the immune function.
The interlectin gene (ITLN1) is expressed in the small bowel and colon, and it is also involved in the recognition of certain microorganisms in the intestine. Other genome-wide association studies have found associations between susceptibility to Crohn disease and polymorphisms in genes that are associated with the intestinal milieu. One such study examined nearly 20,000 single nucleotide polymorphisms in 735 individuals with Crohn disease. [21] An association was found with the ATG16L1 gene, which encodes the autophagy-related 16-like protein, which is involved in the autophagosome pathway that processes intracellular bacteria. [21]
Single nucleotide polymorphisms in other autophagy genes have also shown association with susceptibility to Crohn disease, such as 2 polymorphisms that flanked the IRGM gene and may exert regulatory control for the gene. [22] Subsequently, there have been a number of other loci implicated in the autophagy pathway that have been associated with Crohn disease. [7]
There is strong support for IBD-susceptibility genes on chromosome 5p13.1, which is a gene desert but does modulate expression of the PTGER4 gene. A murine PTGER4 knockout model has significant susceptibility to severe colitis. [23] A large genomic study of multiple diseases confirmed many of the findings that were found in earlier studies, as well as several additional loci of interest for Crohn disease. [24]
Disruption of a homologous gene in a murine model resulted in defective development of the intestine. [25] It was hypothesized that changes in the expression of this gene could alter the migration of lymphocytes in the intestine and change its inflammatory response. The last locus discussed in this model is immediately upstream of the PTPN2 on chromosome 18p11 and encodes a T cell protein tyrosine phosphatase, which is a negative regulator of inflammation. [25]
Ulcerative colitis
The genetic predisposition for ulcerative colitis appears to be lesser in magnitude than Crohn disease but consists of a set of genetic susceptibilities that shows significant overlap with Crohn disease. One genome-wide association study found a previously unknown susceptibility locus at ECM1 and also showed several risk loci that were common to both ulcerative colitis and Crohn disease. [26] Genes that confer risk for both diseases appear to influence the immune milieu of the intestine, whereas the genes that influence only Crohn disease appear to be involved mainly in autophagy. [26, 27]
Additional susceptibility loci for ulcerative colitis have been found on 1p36 and 12q15. The 1p36 single nucleotide polymorphism is near the PLA2G2E gene, which is involved in releasing arachidonic acid from membrane phospholipids, leading to other proinflammatory lipids. The first 12q15 signal is located near the interferon (IFN)-gamma, interleukin (IL)-26, and IL-22 genes, whereas the second 12q15 signal is located in IL-26 gene. These genes play roles in the immune response to pathogens as well as the tissue inflammation processes. [28]
Data suggest that genetic influences increase the risk for one form of IBD while decreasing the risk for another. In a Japanese population, the HLA-Cw*1202-B*5201-DRB1*1502 haplotype increases the risk for ulcerative colitis but reduces the risk for Crohn disease. [29] This finding has not been replicated in other ethnic groups. However, as the authors noted, the human leukocyte antigen (HLA) type in question in this study is relatively common in the Japanese population but relatively rare in European populations. They suggest that the HLA type will favor a T-helper immune response, predisposing toward ulcerative colitis, as opposed to an IFN-predominant response, predisposing more toward Crohn disease. [29]
Smoking
The risk of developing ulcerative colitis is higher in nonsmokers and former smokers than in current smokers. The onset of ulcerative colitis occasionally appears to coincide with smoking cessation; however, this does not imply that smoking would improve the symptoms of ulcerative colitis. There has been limited success with the use of nicotine patches. Crohn disease patients have a higher incidence of smoking than the general population, and smoking appears to lessen the response to medical therapy.
Epidemiology
United States statistics
Before 1960, the incidence of ulcerative colitis was several times higher than that of Crohn disease. More recent data suggest that the incidence of Crohn disease is approaching that of ulcerative colitis.
Annually, an estimated 700,000 physician visits and 100,000 hospitalizations are due to IBD. [30] Approximately 1-2 million people in the United States have ulcerative colitis or Crohn disease, with an incidence of 70-150 cases per 100,000 individuals. [31, 32] In persons of European descent in Olmstead County, Minnesota, the incidence of ulcerative colitis was 7.3 cases per 100,000 people per year, with a prevalence of 116 cases per 100,000 people; the incidence of Crohn disease was 5.8 cases per 100,000 people per year, with a prevalence of 133 cases per 100,000 people. [33, 34]
Racial, sexual, and age-related differences
The incidence and prevalence of inflammatory bowel disease (IBD) among Americans of African descent is estimated to be the same as the prevalence among Americans of European descent, with the highest rates in the Jewish populations of middle European extraction. [35] There is a higher prevalence along a north-south axis in the United States [36] and in Europe, [37] although trends show that the gap is narrowing.
The male-to-female ratio is approximately 1:1 for ulcerative colitis and Crohn disease, with females having a slightly greater incidence. Both diseases are most commonly diagnosed in young adults (ie, late adolescence to the third decade of life).
The age distribution of newly diagnosed IBD cases is bell-shaped; the peak incidence occurs in people in the early part of the second decade of life, with the vast majority of new diagnoses made in people aged 15-40 years. A second, smaller peak in incidence occurs in patients aged 55-65 years and is increasing. Approximately 10% of IBD patients are younger than 18 years.
International statistics
The highest rates of IBD are assumed to be in developed countries, and the lowest are considered to be in developing regions; colder-climate regions and urban areas have a greater rate of IBD than those of warmer climates and rural areas. Internationally, the incidence of IBD is approximately 0.5-24.5 cases per 100,000 person-years for ulcerative colitis and 0.1-16 cases per 100,000 person-years for Crohn disease. [30] Overall, the prevalence for IBD is 396 cases per 100,000 persons annually. [30]
A review of IBD reported that the prevalence of Crohn disease in North America was 319 per 100,000 persons, whereas in Europe, it was 322 per 100,000 persons. [31] Prevalence rates for ulcerative colitis were 249 per 100,000 persons in North America and 505 per 100,000 persons in Europe. The annual incidence of Crohn disease was 20.2 per 100,000 person-years in North America, 12.7 per 100,000 person-years in Europe, and 5.0 per 100,000 person-years in Asia and the Middle East, whereas incidence rates of ulcerative colitis were 19.2 per 100,000 person-years in North America, 24.3 per 100,000 person-years in Europe, and 6.3 per 100,000 person-years in Asia and the Middle East. Time-trend analyses showed statistically significant increases in the incidence of IBD over time. [31]
Prognosis
The standardized mortality ratio for inflammatory bowel disease (IBD) ranges from approximately 1.4 times the general population (Sweden) to 5 times the general population (Spain). Most of this increase appears to be in the Crohn disease population; the ulcerative colitis population appears to have the same mortality rate as the general population. [38]
The majority of studies indicate a small but significant increase in mortality associated with IBD. [39] A frequent cause of death in persons with IBD is the primary disease [40] ; infections and COPD/respiratory illness are other major causes of death. [41] IBD is not a risk factor for cardiovascular mortality. [42]
Patients with IBD are more prone to the development of malignancy. Persons with Crohn disease have a higher rate of small bowel malignancy. Patients with pancolitis, particularly ulcerative colitis, are at a higher risk of developing colonic malignancy after 8-10 years of disease. The current standard of practice is to screen patients with colonoscopy at 1-2 year intervals once they have had the disease for greater than 10 years.
A comprehensive discussion regarding the diagnosis, management, and surveillance of colorectal cancer in patients with IBD is beyond the scope of this article. For more information, see the following two guidelines:
-
Colonoscopic surveillance for prevention of colorectal cancer in people with ulcerative colitis, Crohn's disease or adenomas. (National Institute for Health and Clinical Excellence (NICE), London). [45]
Morbidity
In addition to long-term, disease-related complications, patients can also experience morbidity from prolonged medical therapy, particularly as a consequence of steroid exposure.
There also appears to be an increased risk for IBD in patients with asthma or chronic obstructive pulmonary disease (COPD). In a population-based retrospective cohort study of 136,178 individuals with asthma and 143,904 individuals with COPD, Brassard and colleagues found a significantly increased incidence of IBD. The average incidences of CD and UC in asthma patients were 23.1 and 8.8/100,000 person-years, respectively. Corresponding figures in COPD patients were 26.2 CD and 17 UC cases/100,000 person-years, respectively. [46, 47]
Compared with the general population, the incidence of CD in asthma and COPD patients was 27% and 55% higher, and the incidence of UC was 30% higher among those with COPD. Among children up to 10 years old in the asthma group and adults aged 50 to 59 in the COPD group, the incidence of CD was more than twice that seen in the general population. [46, 47]
Psychologic morbidity affects patients with IBD, especially younger patients, and are typically associated with depression and anxiety symptoms but also exhibit externalizing behaviors. [48] Risk factors for psychologic morbidity appear to include increased disease severity, lower socioeconomic status, use of corticosteroids, parental stress, and older age at diagnosis. [48]
Ulcerative colitis
The average patient with ulcerative colitis has a 50% probability of having another flare during the next 2 years; however, patients may have only one flare over 25 years, and others may have almost persistently active disease. A small percentage of patients with ulcerative colitis have a single attack and no recurrence. Typically, remissions and exacerbations are characteristic of this disease, with acute attacks lasting weeks to months.
Patients with ulcerative colitis limited to the rectum and sigmoid have a 50% chance of progressing to more extensive disease over 10 years [49] and a 7.5% rate of colectomy over 5 years. [50] Approximately 10% of patients presenting with proctitis will develop a pancolitis. [49, 50]
Surgical resection for ulcerative colitis is considered “curative” for this disease, although patients may experience symptoms related to the ileal pouch (J-pouch), including acute and chronic pouchitis. Pouchitis is far more common in patients who have had a colectomy for ulcerative colitis than in those who have had a colectomy for familial adenomatous polyposis.
Beyond 8-10 years after diagnosis, the risk of colorectal cancer increases by 0.5%-1.0% per year. Surveillance colonoscopies with random biopsies reduce mortality from colorectal cancer in patients with ulcerative colitis by allowing the detection of low- or high-grade dysplasia and early stage carcinoma.
Crohn disease
The clinical course of Crohn disease is much more variable than that of ulcerative colitis, and it is dependent on the anatomic location and extent of the disease. Periodic remissions and exacerbations are the rule in Crohn disease. The relapse rate over 10 years is 90%, and the cumulative probability of requiring surgery over 10 years is approximately 38%. Terminal ileum location, fistulizing, and structuring disease are all independent risk factors for subsequent surgery. [51]
A review of the literature indicates that approximately 80% of patients who are in remission for one year will remain in remission over subsequent years. [52] Patients with active disease in the past year have a 70% chance of having clinical disease activity in the following year. Approximately 20% of patients will have annual relapses, and 13% will have a course free of relapses. Less than 5% of patients with Crohn disease will have continually active disease. [52]
Surgery for Crohn disease is generally performed for complications (eg, stricture, stenosis, obstruction, fistula, bleeding, or abscess). Surgical intervention is an important treatment option for Crohn disease, but patients should be aware that it is not curative and that disease recurrence after surgery is high, mimicking the original disease pattern at the site of the surgical anastomosis.
Recurrence of perianal fistulas after medical or surgical treatment is common (59%-82%). [52] In one study, one year after surgery for Crohn disease, 20%-37% of patients had symptoms suggestive of clinical recurrence, and endoscopic evidence of recurrent inflammation was in the neoterminal ileum in 48%-93% of patients. [53]
Overall, the patient's quality of life with Crohn disease is generally lower than that of individuals with ulcerative colitis. Data suggest that in persons with Crohn colitis involving the entire colon, the risk of developing malignancy is equal to that in persons with ulcerative colitis; however, the risk is much smaller (albeit poorly quantified) in most patients with Crohn disease primarily involving the small bowel. Intestinal cancer may become a more important long-term complication in patients with Crohn disease because of longer survival.
Studies support evidence that specific CARD15 mutations are associated with the intestinal location of the disease, as well as course and prognosis, [8] and are correlated with the propensity for developing ileal strictures and with an early onset of disease.
Complications of IBD disease
Intestinal complications
IBD can be associated with several gastrointestinal complications, including risk of hemorrhage, perforation, strictures, and fistulas—as well as perianal disease and related complications, such as perianal or pelvic abscesses, toxic megacolon (complicating acute severe colitis), and malignancy (colorectal cancer, cholangiocarcinoma complicating primary sclerosing cholangitis).
Extraintestinal complications
Extraintestinal complications occur in approximately 20%-25% of patients with IBD. [1] In some cases, they may be more symptomatic than the bowel disease itself. These include osteoporosis (usually a consequence of prolonged corticosteroid use), hypercoagulability resulting in venous thromboembolism, anemia, gallstones, primary sclerosing cholangitis, aphthous ulcers, arthritis, iritis (uveitis) and episcleritis, and skin complications (pyoderma gangrenosum, erythema nodosum).
Table 1, below, summarizes the rates of the most common extraintestinal complications in patients with IBD the United States and Europe.
Table 1. Common Extraintestinal Complications of IBD in US and Europe [54] (Open Table in a new window)
Complication |
Prevalence |
Scleritis |
18% |
Anterior uveitis |
17% |
Gall stones (particularly in Crohn disease) |
13%-34% |
Inflammatory arthritis |
10%-35% |
Anemia |
9%-74% |
Aphthous stomatitis |
4%-20% |
Osteoporosis |
2%-20% |
Erythema nodosum |
2%-20% |
Source: Larson S, Bendtzen K, Nielsen OH. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, diagnosis and management. Ann Med. 2010;42:97-114. |
|
The Swiss National IBD Cohort Study also demonstrated the risks of extraintestinal complications of IBD; their results are summarized in Table 2, below. [55] The risk factors of having complications included family history and active disease observed for Crohn disease only; no significant risk factors were noted in patients with ulcerative colitis. [55]
Table 2. Extraintestinal Complications of IBD in Swiss Patients [55] (Open Table in a new window)
Complication |
Crohn Disease |
Ulcerative Colitis |
Arthritis |
33% |
4% |
Aphthous stomatitis |
10% |
4% |
Uveitis |
6% |
3% |
Erythema nodosum |
6% |
3% |
Ankylosing spondylitis |
6% |
2% |
Psoriasis |
2% |
1% |
Pyoderma gangrenosum |
2% |
2% |
Primary sclerosing cholangitis |
1% |
4% |
Source: Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110-9. |
||
Patient Education
Because inflammatory bowel disease (IBD) is a chronic, often lifelong disease that is frequently diagnosed in young adulthood, increasing patient knowledge improves medical compliance and assists in the management of symptoms.
Encourage the patient to join an IBD support group, such as the Crohn's & Colitis Foundation of America. This foundation can provide educational materials for patients and educational brochures for physicians.
Crohn's & Colitis Foundation of America
733 Third Avenue
Suite 510
New York, NY 10017
Phone: 800-932-2423
E-mail: info@ccfa.org
-
Inflammatory bowel disease. Severe colitis noted during colonoscopy in a patient with inflammatory bowel disease. The mucosa is grossly denuded, with active bleeding noted. The patient had her colon resected very shortly after this view was obtained.
-
Inflammatory bowel disease. Stricture in the terminal ileum noted during colonoscopy in a patient with inflammatory bowel disease. This image depicts a narrowed segment visible upon intubation of the terminal ileum with the colonoscope. Relatively little active inflammation is present, indicating that this is a cicatrix stricture.
-
Inflammatory bowel disease. Enteroenteric fistula noted on a small bowel series of x-ray films in a patient with inflammatory bowel disease. The narrow-appearing segments filled out relatively normally on subsequent films. Note that barium is just starting to enter the cecum in the right lower quadrant (viewer's left), but the barium has also started to enter the sigmoid colon toward the bottom of the picture, thus indicating the presence of a fistula from the small bowel to the sigmoid colon.
-
Inflammatory bowel disease. The table distinguishes features of Crohn disease (CD) and ulcerative colitis (UC).
-
Inflammatory bowel disease. Toxic megacolon. Courtesy of Dr. Pauline Chu.
-
Inflammatory bowel disease. Early pyoderma gangrenosum, before skin breakdown. Medial aspect of the right ankle in a patient with inflammatory bowel disease. Same day and same patient as in the next image.
-
Inflammatory bowel disease. Pyoderma gangrenosum. Courtesy of Dr. Gene Izuno.
-
Inflammatory bowel disease. Severe advanced pyoderma gangrenosum of the medial aspect of the left ankle in a patient with inflammatory bowel disease.
-
Inflammatory bowel disease. Crohn disease involving the terminal ileum. Note the "string sign" in the right lower quadrant (viewer's left).
-
Inflammatory bowel disease. Increased postrectal space is a known feature of ulcerative colitis.
-
Inflammatory bowel disease. Plain abdominal radiograph of a patient with known ulcerative colitis who presented with an acute exacerbation of his symptoms. This image shows thumbprinting in the region of the splenic flexure of the colon.
-
Inflammatory bowel disease. Double-contrast barium enema study shows pseudopolyposis of the descending colon in a patient with ulcerative colitis.
-
Inflammatory bowel disease. Plain abdominal radiograph in a 26-year-old with a 10-year history of ulcerative colitis shows a long stricture/spasm of the ascending colon/cecum (<i>arrow</i>). Note the pseudopolyposis in the descending colon.
-
Inflammatory bowel disease. This single-contrast enema study in a patient with total colitis shows mucosal ulcers with a variety of shapes, including collar-button ulcers, in which undermining of the ulcers occurs, and double-tracking ulcers, in which the ulcers are longitudinally oriented.
-
Inflammatory bowel disease. This double-contrast barium enema study shows total colitis. Note the granular mucosa in the cecum/ascending colon and multiple strictures in the transverse and descending colon in a patient with a more than a 20-year history of ulcerative colitis.
-
Inflammatory bowel disease. Inflamed colonic mucosa demonstrating pseudopolyps in a patient with ulcerative colitis.
-
Inflammatory bowel disease. Chronic architectural changes in ulcerative colitis. Note the crypt branching and irregularity of size and shape, with an increase in chronic inflammatory cells in the lamina propria.
-
Inflammatory bowel disease. High-power view of a crypt abscess in ulcerative colitis shows the crypt to be dilated and filled with neutrophils and debris.
-
Inflammatory bowel disease. Chronic architectural changes in ulcerative colitis. Note the trifid crypt.
-
Inflammatory bowel disease. Basal plasmacytosis in ulcerative colitis. Plasma cells separate the crypt bases from the muscularis mucosae.
-
Inflammatory bowel disease. Low-power image of a colon biopsy specimen in a patient with ulcerative colitis illustrates changes limited to the mucosa. These changes include chronic alterations of the crypt architecture and an increase in chronic inflammatory cells in the lamina propria.
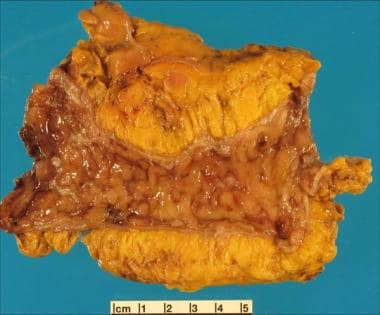
-
Inflammatory bowel disease. Bowel-wall thickening and foreshortening are apparent in this specimen from a colectomy for ulcerative colitis. In addition, the mucosa is hyperemic, with focal nodularity and ulceration.
-
Inflammatory bowel disease. Another gross specimen illustrating ulcerative colitis.
-
Inflammatory bowel disease. This is an example of low-grade glandular dysplasia in a patient with longstanding ulcerative colitis. Note the loss of mucin, nuclear hyperchromasia, and nuclear pseudostratification. See the next image.
-
Inflammatory bowel disease. High-grade dysplasia in the same patient as the previous image. There is significant cytologic atypia, with rounding of the nuclei and a greater degree of pseudostratification.
-
Inflammatory bowel disease. Histologic section from another location in the same patient as described in the previous image. This field shows glands that are suspicious for invasive carcinoma.
-
Inflammatory bowel disease. Computed tomography scan depicting Crohn disease in the fundus of the stomach.
-
Inflammatory bowel disease. Double-contrast barium enema study demonstrates marked ulceration, inflammatory changes, and narrowing of the right colon in a patient with Crohn colitis.
-
Inflammatory bowel disease. Cobblestoning in Crohn disease. Spot views of the terminal ileum from a small bowel follow-through study demonstrates linear longitudinal and transverse ulcerations that create a cobblestone appearance. Also, note the relatively greater involvement of the mesenteric side of the terminal ileum and the displacement of the involved loop away from the normal small bowel secondary to mesenteric inflammation and fibrofatty proliferation.
-
Inflammatory bowel disease. Crohn disease involving the terminal ileum. Note the "string sign" in the right lower quadrant (viewer's left).
-
Inflammatory bowel disease. This computed tomography scan from a patient with terminal ileal Crohn disease shows an enteroenteral fistula (arrow) between loops of diseased small intestine.
-
Inflammatory bowel disease. A teenage patient with Crohn disease underwent a contrast-enhanced upper gastrointestinal computed tomography study with small-bowel follow-through. Several loops of small bowel are in the pelvis. Note there is a loop of distal bowel with a thickened wall (solid arrow), which is contrasted with a less involved loop of bowel in which the intestinal wall is not thickened at all (dotted arrow).
-
Inflammatory bowel disease. Computed tomography scan depicting Crohn disease in the fundus of the stomach.
-
Inflammatory bowel disease. This colonoscopic image of a large ulcer and inflammation of the descending colon in a 12-year-old boy with Crohn disease.
-
Inflammatory bowel disease. This laparoscopic view depicts creeping fat along the mesentery of the terminal ileum in a patient with Crohn disease.
-
Inflammatory bowel disease. Cobblestone change of the mucosa of the terminal ileum in a patient with Crohn disease. Communicating fissures and crevices in the mucosa separate islands of more intact, edematous epithelium.
-
Inflammatory bowel disease. Fat wrapping on the serosal surface of the terminal ileum in Crohn disease. Fat wrapping often correlates directly with underlying strictures, stenosis, or areas of previous transmural inflammation.
-
Inflammatory bowel disease. Colonic granuloma in a patient with Crohn disease (arrow). Hematoxylin-eosin staining. Courtesy of Dr E. Ruchelli.
-
Inflammatory bowel disease. A deep knifelike, fissuring, transmural ulcer in Crohn disease is shown in this histologic image.
-
Inflammatory bowel disease. Another example of a deep, fissuring ulcer in a patient with Crohn disease. Note the increase in submucosal inflammation and scattered lymphoid aggregates.
-
Inflammatory bowel disease. Prominent lymphoid aggregates and granuloma in the muscularis propria and pericolic fat of patient with Crohn disease. The inflammation extends through the full thickness of the bowel wall.
-
Inflammatory bowel disease. A crypt abscess demonstrating active, neutrophilic inflammation in Crohn disease.
-
Inflammatory bowel disease. Granuloma in the mucosa in a Crohn disease patient.
-
Inflammatory bowel disease. Double-contrast barium enema study shows changes of ulcerative colitis disease. Note the granular mucosa.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Treatment
- Approach Considerations
- Symptomatic Therapy/Supportive Care
- Overview of Therapy
- Aminosalicylates
- Antibiotics
- Corticosteroids
- Immunomodulators
- Clinical Trial Agents
- Inpatient Management
- Management of Refractory Disease
- Management in Remission
- Management of the Older IBD Patient
- Surgical Intervention
- Diet, Lifestyle Modifications, and Activity
- Reproduction and Pregnancy
- Consultations
- Show All
- Guidelines
- Guidelines Summary
- British Society of Gastroenterology Guidelines (BSG) (2019)
- European Crohn's and Colitis Organisation Guidelines on Medical Treatment (ECCO) (2019)
- European Crohn's and Colitis Organisation Guidelines on Surgical Management (ECCO) (2019)
- American Gastroenterological Association Guidelines (AGA) (2020)
- Resources
- Show All
- Medication
- Medication Summary
- 5-Aminosalicylic Acid Derivatives
- Antibiotics, Other
- Corticosteroids
- Immunosuppressants
- TNF Inhibitors
- Alpha 4 Integrin Inhibitors
- Histamine H2 Antagonists
- Proton Pump Inhibitors
- Antidiarrheals
- Anticholinergic, Antispasmodic Agents
- Monoclonal Antibodies
- Corticosteroids, Rectal
- DMARDs, JAK Inhibitors
- Bile Acid Sequestrants
- Show All
- Questions & Answers
- Media Gallery
- Tables
- References










