Practice Essentials
Bullous pemphigoid is a chronic, inflammatory, subepidermal, blistering disease. If untreated, it can persist for months or years, with periods of spontaneous remissions and exacerbations. The disease can be fatal, particularly in patients who are debilitated.
Signs and symptoms
Bullous pemphigoid may present with several distinct clinical presentations, as follows:
-
Generalized bullous form: The most common presentation; tense bullae arise on any part of the skin surface, with a predilection for the flexural areas of the skin
-
Vesicular form: Less common than the generalized bullous type; manifests as groups of small, tense blisters, often on an urticarial or erythematous base
-
Vegetative form: Very uncommon, with vegetating plaques in intertriginous areas of the skin, such as the axillae, neck, groin, and inframammary areas
-
Generalized erythroderma form: This rare presentation can resemble psoriasis, generalized atopic dermatitis, or other skin conditions characterized by an exfoliative erythroderma
-
Urticarial form: Some patients with bullous pemphigoid initially present with persistent urticarial lesions that subsequently convert to bullous eruptions; in some patients, urticarial lesions are the sole manifestations of the disease
-
Nodular form: This rare form, termed pemphigoid nodularis, has clinical features that resemble prurigo nodularis, with blisters arising on normal-appearing or nodular lesional skin
-
Acral form: In childhood-onset bullous pemphigoid associated with vaccination, the bullous lesions predominantly affect the palms, soles, and face
-
Infant form: In infants affected by bullous pemphigoid, the blisters tend to occur frequently on the palms, soles, and face, affecting the genital areas rarely; 60% of these infant patients have generalized blisters [1]
See Clinical Presentation for more detail.
Diagnosis
To establish a diagnosis of bullous pemphigoid, the following tests should be performed:
-
Histopathologic analysis: From the edge of a blister; the histopathologic examination demonstrates a subepidermal blister; the inflammatory infiltrate is typically polymorphous, with an eosinophil predominance; mast cells and basophils may be prominent early in the disease course
-
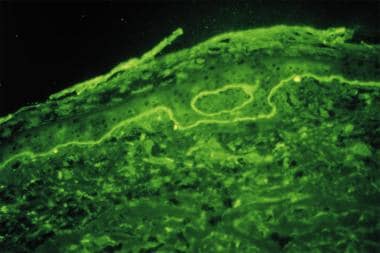
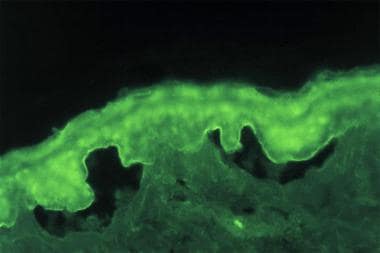
Indirect immunofluorescence (IDIF) studies: Performed on the patient’s serum, if the DIF result is positive (see the image below)
DIF tests in patients with bullous pemphigoid usually demonstrate immunoglobulin G (IgG) and complement C3 deposition in a linear band at the dermal-epidermal junction, with IgG in salt-split skin found on the blister roof (epidermal side of split skin).
IDIF studies document the presence of circulating IgG autoantibodies in the patient's serum that target the skin basement membrane component. Seventy percent of patients with bullous pemphigoid have circulating autoantibodies that bind to split skin.
See Workup for more detail.
Management
As in other autoimmune bullous diseases, the goal of therapy in bullous pemphigoid is as follows:
-
Decrease blister formation
-
Promote healing of blisters and erosions
-
Determine the minimal dose of medication necessary to control the disease process
The most commonly used medications for bullous pemphigoid are anti-inflammatory agents (eg, corticosteroids, tetracyclines, dapsone) and immunosuppressants (eg, azathioprine, methotrexate, mycophenolate mofetil, cyclophosphamide). [4, 5] Initial treatment with doxycycline was found to be effective and was associated with a lower incidence of adverse effects compared with prednisone. [6, 7, 8]
Most patients affected with bullous pemphigoid require therapy for 6-60 months, after which many patients experience long-term remission of the disease. However, some patients have long-standing disease requiring treatment for years.
Most mortality associated with bullous pemphigoid occurs secondary to the effects of medications used in treatment. For example, the population at risk for bullous pemphigoid is at an increased risk for comorbid conditions, such as hypertension, diabetes mellitus, and heart disease, which treatment may exacerbate.
See Treatment and Medication for more detail.
Background
Bullous pemphigoid is a chronic, autoimmune, subepidermal, blistering skin disease that rarely involves mucous membranes. Bullous pemphigoid is characterized by the presence of immunoglobulin G (IgG) autoantibodies specific for the hemidesmosomal bullous pemphigoid antigens BP230 (BPAg1) and BP180 (BPAg2). BP antigen 2 is the usual pathogenic antibody. Occasionally, sublamina densa deposits are noted, related to anti-p200 antibody.
In spontaneous animal models, bullous pemphigoid has been reported to occur in dogs (canine) [9, 10] and horses (equine). [11] Bullous pemphigoid has been found to occur in domestic cats (feline) and Yucatan minipigs (porcine). [12]
In experimental animal models, passive transfer of antibodies to mouse BPAg2 causes blistering in newborn mice similar to that seen in humans. Active induction of anti-BPAg1 antibodies in rabbits enhances inflammation and deposition of immunoreactants at the basement membrane but does not result in spontaneous blistering. [13, 14, 15]
In canine bullous pemphigoid, histologic analysis reveals a subepidermal blistering process with prominent eosinophil infiltration identical to the classic pathology of humans. Similar findings have been observed in feline, [16] porcine, and equine bullous pemphigoid. [11]
As in humans with bullous pemphigoid, the sera from dogs with bullous pemphigoid bind to the epidermal roof of salt-split skin and BP180. The antigenic epitopes of BP180 identified by the canine bullous pemphigoid IgG map to the same epitopes as human bullous pemphigoid autoantibodies. Similar findings were observed in cats, [16] pigs, and horses [11] with bullous pemphigoid.
Pathophysiology
IgG autoantibodies bind to the skin basement membrane in patients with bullous pemphigoid. The binding of antibodies at the basement membrane activates complement and inflammatory mediators. Activation of the complement system is thought to play a critical role in attracting inflammatory cells to the basement membrane. These inflammatory cells are postulated to release proteases, which degrade hemidesmosomal proteins and lead to blister formation. Eosinophils are characteristically present in human patients' blisters as demonstrated by histopathologic analysis, although their presence is not an absolute diagnostic criterion.
The precise role of bullous pemphigoid antigens in the pathogenesis of bullous pemphigoid is not completely clear. BPAg1 (BP230) is an intracellular component of the hemidesmosome; BPAg2 (BP180, type XVII collagen) is a transmembranous protein with a collagenous extracellular domain. [17] Passive transfer experiments in newborn mice have demonstrated that rabbit antibodies against mouse BPAg2 can induce subepidermal blisters similar to those observed in patients with bullous pemphigoid. However, the eosinophil infiltration that is frequently observed in human bullous pemphigoid lesional skin was not detected in the passive transfer experimental model. [18] Furthermore, anti-BP180 NC16A domain autoantibodies purified from patients with bullous pemphigoid are capable of inducing dermal-epidermal separation in cryosections of normal human skin. [19]
Studies from 2006 on autoreactive T and B cells from 35 patients with acute-onset bullous pemphigoid revealed that the percentage of T- cell and B-cell reactivity from these bullous pemphigoid patients against the BPAg2 is much higher than that against BPAg1, further suggesting a more prominent role of BPAg2 in disease development. [20]
Serum levels of autoantibodies against BPAg2 are reportedly correlated with disease activity in some studies. [21] Induction of antibodies against BPAg1 in rabbits does not induce primary blistering, but it can enhance the inflammatory response at the basement membrane. The role of autoantibodies specific for bullous pemphigoid antigens in the initiation and the perpetuation of disease is unknown.
Although BPAg2 has been identified as the major antigen involved with bullous pemphigoid disease development, in 2005, autoantibodies against alpha 6 integrin [22] and laminin-5, [23] 2 other skin basement membrane components, were identified in human patients affected by bullous pemphigoid.
Although no perfect active experimental model is available currently, an active animal model was generated by transferring splenocytes from wide-type mice that had been immunized by grafting human BP180-transgenic mouse skin into Rag-2(-/-)/BP180-humanized mice. [24] The recipient immunodeficient mice developed antihuman BP180 antibodies, manifested with blisters that are consistent with the clinical, histological, and immunopathological features of human bullous pemphigoid, except eosinophil infiltration. [24] In addition, the autoantibody response can be induced in healthy BALB/c mice by immunizing the mice with synthetic peptides of the mouse type XVII collagen NC16A domain, the target region of autoantibodies in human patients affected with bullous pemphigoid. [25]
Eotaxin, an eosinophil-selective chemokine, is strongly expressed in the basal layer of the epidermis of lesional bullous pemphigoid skin and parallels the accumulation of eosinophils in the skin basement membrane zone area. It may play a role in the recruitment of eosinophils to the skin basement membrane area.
Other cytokines and chemokines have also been studied in bullous pemphigoid. Interleukin 16, a major chemotactic factor responsible for recruiting CD4+ helper T cells to the skin and for inducing functional interleukin 2 receptors for cellular activation and proliferation, was found to be expressed strongly by epidermal cells and infiltrating CD4+ T cells in lesional bullous pemphigoid skin. Significantly higher levels of interleukin 16 were detected in sera and blisters of bullous pemphigoid patients compared with healthy subjects. These data (reported in 2004 and involved 39 bullous pemphigoid patients with active disease) suggest a role of interleukin 16 in bullous pemphigoid development. [26]
In other study of 27 bullous pemphigoid patients (reported in 2006), serum levels of monokine induced by interferon gamma (MIG, a Th1-type chemokine) and serum levels of CCL17 and CCL22 (Th2-type chemokines) were significantly increased in bullous pemphigoid patients compared with healthy subjects. [27]
Matrix metalloproteinase (MMP)–2, MMP-9, and MMP-13 were significantly increased in lesional bullous pemphigoid skin compared with that of healthy skin, with T cells comprising the majority of MMP cellular sources. These data (reported in 2006) suggest a role of MMP in the blistering of bullous pemphigoid. [28]
In another study of 39 bullous pemphigoid patients (reported in 2006), a cytokine named BAFF (B-cell activating factor belonging to the tumor necrosis factor family) that functions to regulate B-cell proliferation and survival was found to be significantly increased in sera of bullous pemphigoid patients compared with healthy subjects, although no significant association was noted between serum BAFF levels and titers of anti-BPAg2 antibodies. [29]
In 2008, a role for IgE class of autoantibodies, particularly those that target BP180, has been established. The higher level of IgE anti-BP180 was correlated with a more severe clinical phenotype. [30]
In an animal model in which C57BL/6 type of mice engrafted with syngeneic mouse skin transgenically expressed human BPAg2 in the epidermal basement membrane zone, antibodies against the human BPAg2 extracellular domain developed first, followed by the occurrence of antibodies to the intracellular domain of the same human BPAg2. Interestingly, the development of later antibodies was associated with the loss of the graft. [31]
IgG autoantibodies from bullous pemphigoid patients are found to deplete cultured keratinocytes of the BPAg2 and weaken cell attachment in vitro, which further supports the pathogenic role of these autoantibodies. [32]
The coagulation cascade is found to be activated in bullous pemphigoid patients, and such activation is found to be correlated with the disease severity and with eosinophilia, suggesting a role of eosinophils in this activation of coagulation, which may contribute to the potential thrombotic risk, as well as inflammation, tissue damage, and blister formation. [33]
A 2010 report of finding anti-BP180 antibodies in unaffected subjects is provides interesting data for further study of the pathogenesis of bullous pemphigoid. [34]
A 2009 report of bullous pemphigoid developed after adalimumab treatment for psoriasis raises some question about whether biologics can play a role in inducing the disease or it may just suggest the association of bullous pemphigoid with psoriasis. [35]
Bullous pemphigoid has been associated with PD-1 inhibitors used as targeted therapy for malignancy. Some patients developed both pemphigoid and keratoacanthomas. [36, 37, 38]
Dipeptidyl peptidase 4 (DPP-4) inhibitors are associated with a risk of developing bullous pemphigoid. [39, 40, 41, 42]
Epidemiology
United States
Bullous pemphigoid is uncommon, and its frequency is unknown.
International
Bullous pemphigoid has been reported to occur throughout the world. In France and Germany, the reported incidence is 6.6 cases per million people per year. In Europe, bullous pemphigoid was identified as the most common subepidermal autoimmune blistering disease.
In a population-based cohort study, the incidence of bullous pemphigoid was found to be 4.3 cases per 100,000 person-years in the United Kingdom. [43]
Race
No racial predilection is apparent.
Sex
The incidence of bullous pemphigoid appears to be equal in men and women.
Age
Bullous pemphigoid primarily affects elderly individuals in the fifth through seventh decades of life, with an average age at onset of 65 years. Bullous pemphigoid of childhood onset has been reported in the literature. It is suggested that the childhood-onset bullous pemphigoid may be more self-limited. [44] One puzzling finding, however, is a report of rising incidence of infant-onset bullous pemphigoid.
Prognosis
Most patients affected with bullous pemphigoid require therapy for 6-60 months, after which many patients experience long-term remission of the disease. However, some patients have long-standing disease requiring treatment for years. Most mortality associated with bullous pemphigoid occurs secondary to the effects of the medications. Data suggest that high serum levels of IgG1 and IgG4 targeting the noncollagenous 16A domain of BP180 correlate with more serious disease and a worse prognosis. [45] Age and the presence of circulating antibodies are also associated with poor outcome. [46]
The population at risk for bullous pemphigoid is at an increased risk for comorbid conditions, such as hypertension, diabetes mellitus, thromboembolism, and heart diseases, which treatment may exacerbate. [47] Bullous pemphigoid may be linked (directly or indirectly) to neurological disorders. [48, 49] An increase in the occurrence of neurological disorders has been reported in patients affected by bullous pemphigoid, relative to the age-matched and sex-matched general population. [50] However, these findings need to replicated in different populations to clarify this proposed relationship.
In a multicenter, prospective cohort in France, a high titer of anti-BPAg2 autoantibodies by ELISA and a positive direct immunofluorescence finding were found to be good indicators of further clinical relapse of bullous pemphigoid and may correlate with overall morbidity and mortality. [51, 52, 53]
Mortality/Morbidity
Bullous pemphigoid is a chronic inflammatory disease. If untreated, the disease can persist for months or years, with periods of spontaneous remissions and exacerbations. In most patients who are treated, bullous pemphigoid remits within 1.5-5 years. Patients with aggressive or widespread disease, those requiring high doses of corticosteroids and immunosuppressive agents, and those with underlying medical problems have increased morbidity and risk of death. Because the average age at onset of bullous pemphigoid is about 65 years, patients with bullous pemphigoid frequently have other comorbid conditions that are common in elderly persons, thus making them more vulnerable to the adverse effects of corticosteroids and immunosuppressive agents.
Bullous pemphigoid may be fatal, particularly in patients who are debilitated. The proximal causes of death are infection with sepsis and adverse events associated with treatment. Patients receiving high-dose corticosteroids and immunosuppressants are at risk for peptic ulcer disease, GI bleeds, agranulocytosis, and diabetes.
Bullous pemphigoid involves the mucosa in 10-25% of patients. Patients who are affected may have limited oral intake secondary to dysphagia. Erosions secondary to rupture of the blisters may be painful and may limit patients' daily living activities. Blistering on the palms and the soles can severely interfere with patients' daily functions.
Bullous pemphigoid lesions typically heal without scarring or milia formation. In a survey of patients conducted in a Midwest United States university medical center, no difference was noted in expected mortality in bullous pemphigoid 223 patients compared with the general population. [54] In a population-based cohort study in the United Kingdom, however, the risk of death for bullous pemphigoid patients was found to be twice as great as that for controls. [43] Furthermore, a Swiss prospective study confirmed a high-case fatality rate, with increased 1-year mortality compared with the expected mortality rate for age-adjusted and sex-adjusted general population. [55]
Patient Education
Patients should avoid trauma to the skin. Patients' skin is fragile from the disease, as well as from the use of topical and systemic steroids. Patients should be educated about their disease and treatments, so that they can report adverse effects to their physicians.
-
Direct immunofluorescence study performed on a perilesional skin biopsy specimen from a patient with bullous pemphigoid detects a linear band of immunoglobulin G deposit along the dermoepidermal junction.
-
Indirect immunofluorescence study performed on salt-split normal human skin substrate with the serum from a patient with bullous pemphigoid detects immunoglobulin G class circulating autoantibodies that bind to the epidermal (roof) side of the skin basement membrane.