Background
Polymorphous light eruption (PMLE) is an acquired disease and is the most common of the idiopathic photodermatoses. First described by Ebstein in 1942 as prurigo aestivalis. PMLE is characterized by recurrent, abnormal, delayed reactions to sunlight, ranging from erythematous papules, papulovesicles, and plaques to erythema multiforme–like lesions on sunlight-exposed surfaces. Within any single patient, only one clinical form is consistently manifested. The word polymorphous in the name refers to the different morphologic presentation of the condition.
PMLE engenders a substantial psychosocial impact. In a review by Richards et al, up to 40% of patients described emotional distress related to PMLE. [1] Women associated more severe consequences linked to PMLE and were more emotionally distressed than men. Patients experience a decrease in quality of life owing to efforts to avoid sun exposure.
Management of PMLE includes strict sun protection. This can be accomplished by using broad-spectrum sunscreens, seeking shade, and wearing protective clothing, including hat wear. Photohardening is beneficial and can be used early in spring to increase tolerance to sun exposure. Topical and systemic immunosuppressants are used for symptom management. Their use should be tailored to each patient where the benefits and risks are weighted.
The possibility that a subset of PMLE called benign summer light eruption (BSLE), which might be milder and might be more ultraviolet (UV)–A driven, has been suggested by an Italian group. [2]
Note the images below.
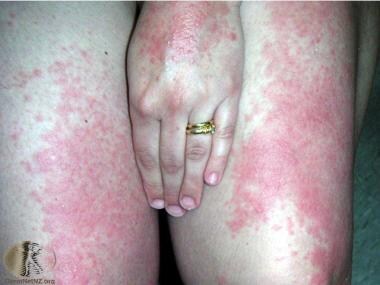 Polymorphous light eruption on the thighs and hand. Courtesy of DermNet New Zealand (http://www.dermnetnz.org/assets/Uploads/reactions/pmle2.jpg).
Polymorphous light eruption on the thighs and hand. Courtesy of DermNet New Zealand (http://www.dermnetnz.org/assets/Uploads/reactions/pmle2.jpg).
Pathophysiology
The etiology of polymorphous light eruption (PMLE) is not fully known, and it is likely to be multifactorial. The appearance in families supports a genetic association. The production of neoantigens, failure to induce apoptosis, poor immune tolerance, delayed hypersensitivity reaction, and skin microbiome dysregulation are important factors in the pathogenesis of PMLE. The action spectrum is primarily UVA light, but can include UVB light. Some patients even react in the visible light spectrum. It has also been shown that UVC can cause PMLE. [3]
PMLE clusters in families, suggesting a genetic component. In a study by Millard et al, they studied 420 pair of adult twins and found that 21% of monozygotic and 18% of dizygotic twins had PMLE. [4] First-degree family history was seen in 12% compared with 4% of unaffected twins. The prevalence of PMLE in first-degree family members is seen in 20.9% of members. [5]
Actinic prurigo (AP) is thought to be a subtype of PMLE, given that they share common pathophysiology. AP tends to persist longer, and lesions can involve the mucosa, including the conjunctiva. Excoriations and scaring are other features of AP not seen in PMLE. HLA-DR4 is strongly associated with AP. [6]
Delayed hypersensitivity reaction of PMLE is supported by the study of timed biopsy samples of PMLE lesions. The CD4 subtype of T cells seen very early after exposure is replaced by CD8 lymphocytes 72 hours after irradiation. [7, 8]
Kölgen et al noted that the reduced expression of tumor necrosis factor-alpha, interleukin (IL)–4, and IL-10 in the UVB-irradiated skin of patients with PMLE. The reduction of these cytokines seems linked to a relative neutropenia and is a manifestation of decreased Langerhans cell migration and reduced TH2 skewing. [9] An impairment of these mechanisms underlying UVB-induced immunosuppression may be important in the pathogenesis of PMLE.
An increase in the IL-1 family of cytokines, in particular (IL-36 gamma), in skin lesions and peripheral blood of PMLE patients indicates an enhanced focal and systemic immune response. This further supports the enhanced immune response upon UV exposure. [10]
A study by Koulu et al assessed 48 subjects (24 patients with PMLE and 24 healthy sex-matched and age-matched controls). [11] The study found similar immunosuppression of contact sensitization to diphenylcyclopropenone due to earlier exposure to solar-simulating UV radiation between both groups. However, among patients with PMLE who were immunosuppressed by UV radiation, only one exhibited immunotolerance to the same allergen 10-24 months later (P = .023). The study concluded that impaired propensity to UV-induced, allergen-specific immunotolerance may promote recurrent PMLE.
Neutrophils may play a role in the development of PMLE. Immunohistochemical analysis by Schornagel et al in 2004 showed a significant decreased neutrophil infiltration in PMLE skin after UVB irradiation compared with healthy case control subjects (P< .05). [12] Intercellular adhesion molecule 1 (ICAM-1) and E-selectin expression on endothelial cells increased in both healthy controls and in the PMLE patients after UVB irradiation. Chemotactic response towards IL-8 and C5a was not different between PMLE patients and healthy controls. The authors concluded that PMLE is marked by an altered immune response resulting in decreased skin infiltration of neutrophils after UVB irradiation.
Apoptotic keratinocyte produce photoneoantigens and failure to clear these antigens leads to an increased immune response. In photoprovocated skin samples of PMLE patients, gene expression of apoptotic cell clearance C1S and SCARB1 are reduced. [13] This, along with the failure in immune tolerance, leads to PMLE skin lesions with light exposure. In fact, this might explain the reduced rate of skin cancer in patients with PMLE. [14, 15]
Regarding microbiome dysregulation, UV-induced changes to the skin microbiome in PMLE patients was proposed as an initiating or provoking factor in the inflammatory cascade, by antimicrobial peptide (AMP) release and activation of the innate immune system. A unique pattern of AMP was seen in PMLE patients when compared with patients with atopic dermatitis, psoriasis, or normal skin. An increase in psoriasin, RNAse7, human beta defensin-2 (HBD‐2), and LL37 are seen in PMLE, similar to psoriasis. However, PMLE patients had a lower level of HBD-3 compared with psoriasis and atopic dermatitis patients. [10, 16]
Intravascular and focal perivascular deposits of fibrin were detected in biopsy samples of PMLE papules. Vascular deposits of C3 and immunoglobulin M (IgM) were noted in a few patients. These findings may suggest that vascular injury with activation of a clotting cascade may play a role in the pathogenesis of PMLE. [17]
In some PMLE lesions induced by UVA, keratinocytes were found to express ICAM-1. [18, 19] ICAM-1 is absent from normal keratinocytes, but it is known to be strongly induced by interferon-gamma. The induction of ICAM-1 on keratinocytes results either from direct effects of UV on the promoter region of the ICAM-1 gene or from indirect effects of interferon-gamma produced by activated lymphocytes aggregating in an underlying PMLE.
The demonstration that the female hormone 17beta-estradiol prevents UV radiation–induced suppression of the contact hypersensitivity response caused by the release of immunosuppressive cytokines (IL-10) from keratinocytes might explain why the risk of PMLE is higher in females than in males and why the risk decreases in women after menopause. [20]
Some have suggested that glutathione S-transferases (GSTs) act to protect against PMLE, but a study of the isoenzymes of the GST genes GSTM1, GSTT1, and GSTP1 found no protective relationship of these isoenzymes to PMLE. [21]
It is possible that the use of tobacco makes PMLE worse. [22]
Etiology
The UVA light spectrum is the most common causative factor in polymorphous light eruption (PMLE). Other wavelengths, including UVB or even visible light, may also induce PMLE in some individuals. PMLE-like lesions have been reported in welders, resulting from exposure to UVC light. [23]
Overall, family history is positive for PMLE in about 15-20% of the patients. However, Native Americans have a hereditary form of PMLE with apparent autosomal dominant inheritance; 75% reveal disease in a family member. [24]
Epidemiology
Frequency
United States
Polymorphous light eruption (PMLE) affects about 10-20% of the US and Western Europe population. [25] This figure is likely to be an underestimate because many patients do not seek medical attention. Additionally, many of the photodermatoses were previously lumped together before their individual pathogeneses were identified. PMLE is now a distinct clinical entity, as are many of the other photodermatoses (eg, solar urticaria, photoallergic dermatitis, hydroa vacciniforme, chronic actinic dermatitis, erythropoietic porphyria, lupus erythematosus).
Benanni et al noted a low incidence of PMLE in renal transplant recipients; this can be attributed to the immunosuppressants used to prevent rejection. [26]
International
Deng et al used a questionnaire to survey 4899 residents (49% men and 51% women) from randomly selected Chinese villages in several counties at different altitudes and with different ethnic majorities. [27] The prevalence of PMLE was 32 (0.65%) in 4899 residents and was 3.8 times higher in women compared with men. At higher elevations, the prevalence of PMLE increased. The mean time of sun exposure for PMLE was 6 h/day. The mean duration of PMLE was 5.8 years. [27]
A report from India in 2013 notes that PMLE is the most common photodermatitis after chronic actinic dermatitis, while hydroa vacciniforme and solar urticaria are uncommon. [28] Furthermore, this report stated lichenoid PMLE and pinpoint papular PMLE are the most common types found in the subcontinent.
Race
PMLE affects all racial skin types. In a Scandinavian/Mediterranean study, PMLE was more common in patients with Fitzpatrick skin type I. The papular variant can involve the face and seems most common in patients with Fitzpatrick skin types III-VI. [29, 30]
In a study of 280 American patients with photodermatoses, [31] one hundred thirty-five (48%) were African Americans, 110 (40%) were white, and 35 (12%) were patients of other races. They noted a statistically significantly higher proportion of African Americans with PMLE compared with whites.
One study of a cohort of 229 patients with photodermatoses reported that 63 (42.2%) were white and 138 (46.6%) were African American and PMLE was present in 54% and 86.2%, respectively (P< .0001) [32] ; thus, the researchers suggested that PMLE occurs more frequently in African Americans.
Sex
PMLE affects females 2-3 times more often than males. However, these data may be skewed because females are more likely to seek medical attention than males.
Age
PMLE usually has an onset in the first three decades of life. Onset during childhood and late adulthood can be seen. Men seem to have later onset of the disease than women.
Studies have reported mean ages of 26.4 years and 37.8 years in their respective cohorts. [33, 34]
Prognosis
Expression of polymorphous light eruption (PMLE) may range from an insignificant, mild rash to severe disease affecting the patient's quality of life. Some PMLE patients experience a less severe reaction with each consecutive year, but many patients have reactions that may worsen with time without appropriate treatment. Each case should be evaluated individually.
PMLE is a chronic condition; the average time to resolution is long and can reach up to 30 years.
Richards et al found that emotional distress attributable to PMLE occurred in greater than 40% of individuals. [1] Women more than men associated more severe consequences with their PMLE and experienced more emotional distress.
-
Photograph of a springtime eruption of polymorphous light eruption in a 6-year-old boy. The eruption has occurred in the spring since the patient was aged 5 years, and it resolves completely with no scarring by mid to late summer. High block sunscreens attenuate but do not prevent the eruption. No itching or pain occurs. Courtesy of Dr. Jeremy F. Harrison, FRCA, FFAEM.
-
Polymorphous light eruption on the thighs and hand. Courtesy of DermNet New Zealand (http://www.dermnetnz.org/assets/Uploads/reactions/pmle2.jpg).
-
Polymorphous light eruption on the chest. Courtesy of DermNet New Zealand (http://www.dermnetnz.org/assets/Uploads/_resampled/FitWzY0MCw0ODBd/WatermarkedWyIyNTg0MSJd/pmle-14.jpg).
-
Polymorphous light eruption on the chest. Courtesy of DermNet New Zealand (http://www.dermnetnz.org/assets/Uploads/_resampled/FitWzY0MCw0ODBd/WatermarkedWyIyNTg0MCJd/pmle-15.JPG).
-
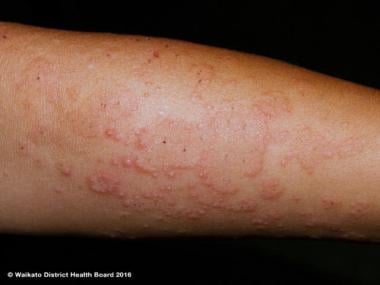
Polymorphous light eruption on the arm. Courtesy of Waikato District Health Board and DermNet New Zealand (http://www.dermnetnz.org/assets/Uploads/_resampled/FitWzY0MCw0ODBd/WatermarkedWyIyNTg0MCJd/pmle-22.JPG).
-
Polymorphous light eruption pathology showing papillary dermal edema. Courtesy of DermNet New Zealand (https://www.dermnetnz.org/assets/Uploads/pathology/e/pmle-figure-1.jpg).
-
Polymorphous light eruption pathology showing papillary dermal edema. Courtesy of DermNet New Zealand (https://www.dermnetnz.org/assets/Uploads/pathology/e/pmle-figure-2.jpg).
-
Polymorphous light eruption pathology showing papillary dermal edema with lymphocytes in the epidermis (exocytosis). Courtesy of DermNet New Zealand (https://www.dermnetnz.org/assets/Uploads/pathology/e/pmle-figure-3.jpg).
-
Polymorphous light eruption pathology. The infiltrate is mainly lymphocytic but there may be intermixed eosinophils, neutrophils, and histiocytes. Courtesy of DermNet New Zealand (https://www.dermnetnz.org/assets/Uploads/pathology/e/pmle-figure-4.jpg).